Structural molecule for affinity and target cell uptake capability of enhanced integrin receptor and application thereof
An integrin receptor, affinity technology, applied in the field of biomedicine, can solve the problems of inability and lack of siRNA uptake and release ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 adopts the following steps:
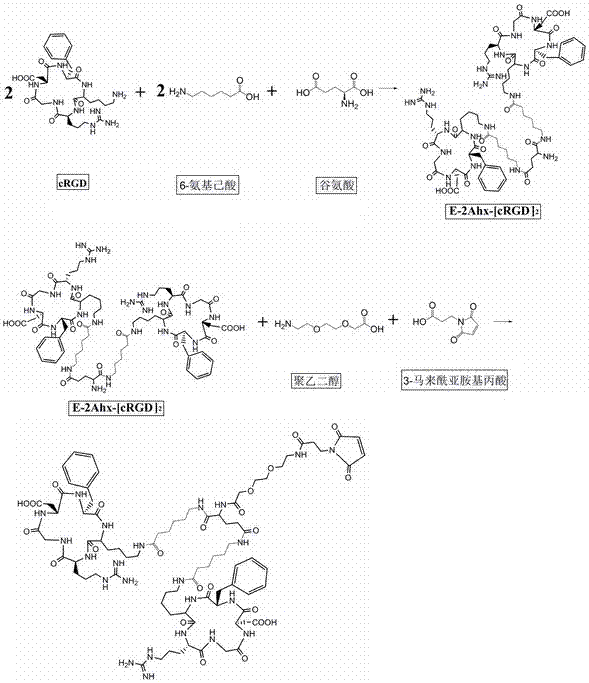
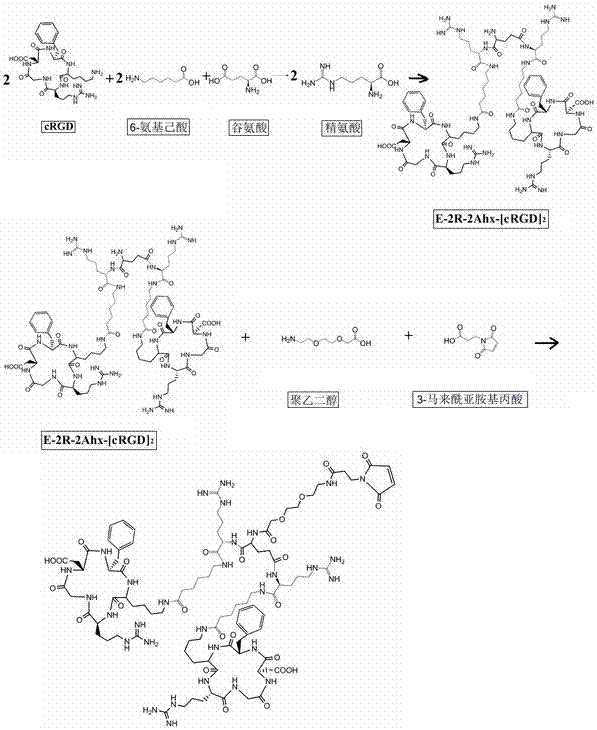
[0052] 1.1. Synthesize different molecular structures E-2Ahx-[cRGD]2-PEG-MPA (biRGD for short) and E-2R-2Ahx-[cRGD2-PEG-MPA (biRGD2 for short) that enhance the targeting affinity and uptake of tumor sites :
[0053] The synthesis steps of biRGD1 and biRGD2 are as follows: figure 1 and figure 2 As shown, the dehydration condensation of lysine in two monovalent cRGD motifs with 6-aminocaproic acid, glutamic acid and / or arginine, 8-amino-3,6-dioxahexanoic acid affords E-2Ahx -[cRGD]2-PEG or E-2R-2Ahx-[cRGD]2-PEG, then the amino group of PEG is dehydrated and condensed with the carboxyl group of 3-maleimidopropionic acid to obtain E-2Ahx-[cRGD]2 - PEG-MPA and E-2R-2Ahx-[cRGD]2-PEG-MPA.
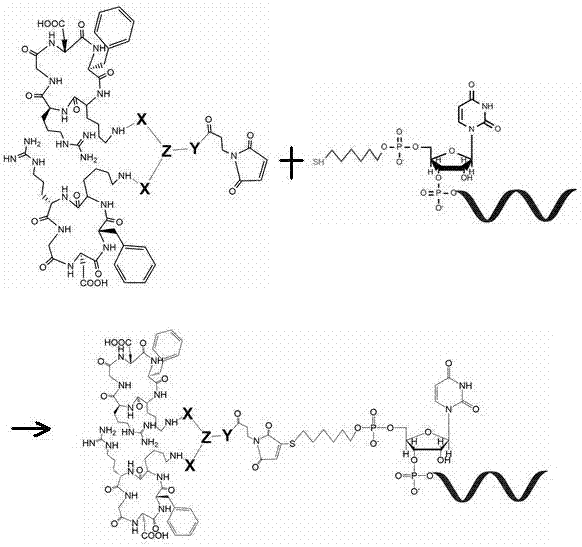
[0054] 1.2. Synthesis of positive-sense strand RNA with thiol (-SH) modification:
[0055] siACTB is a siRNA molecule that silences human β-actin mRNA and has undergone conventional stability modification; siNC is a siRNA molecule that has no...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com