Mometasone furoate suspension nasal spray composition
A technology of mometasone furoate and nasal spray, which is applied in drug combination, steroids, and drug delivery, and can solve the problems of not including mometasone furoate nasal spray
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Invention Example 1 Preparation of Mometasone Furoate Form M
[0038] Dissolve 1 g of commercially available mometasone furoate in 50 ml of acetonitrile, heat until dissolved, then evaporate the solvent, crystals are precipitated, then filter and dry to obtain crystal form M of mometasone furoate.
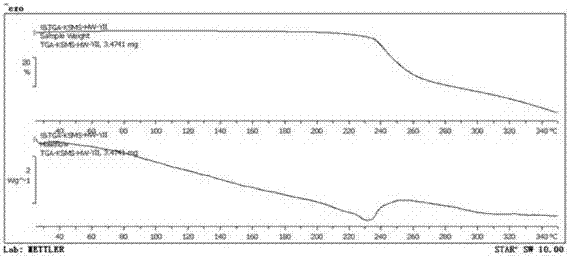
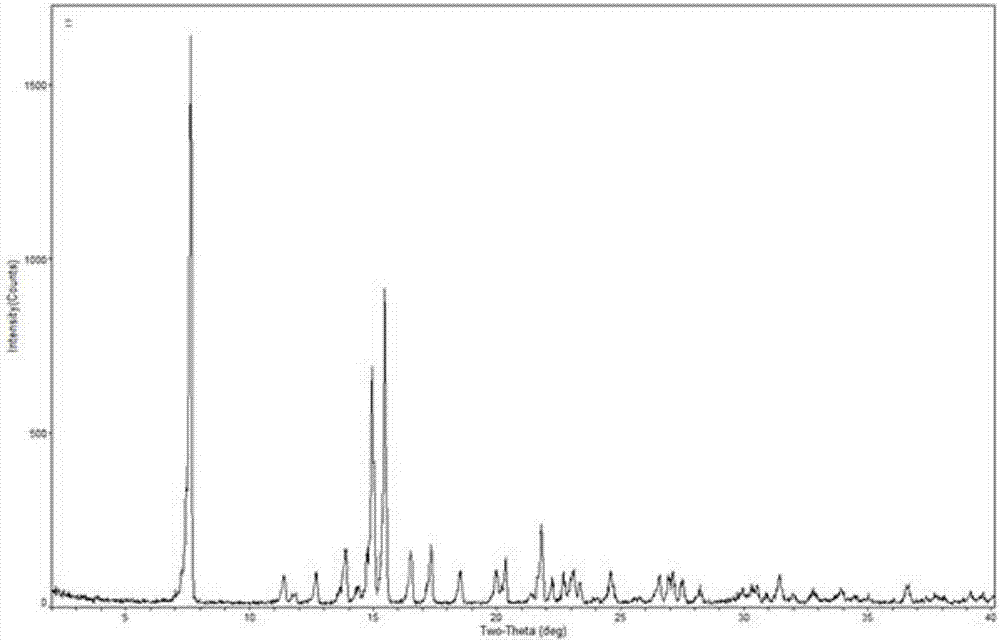
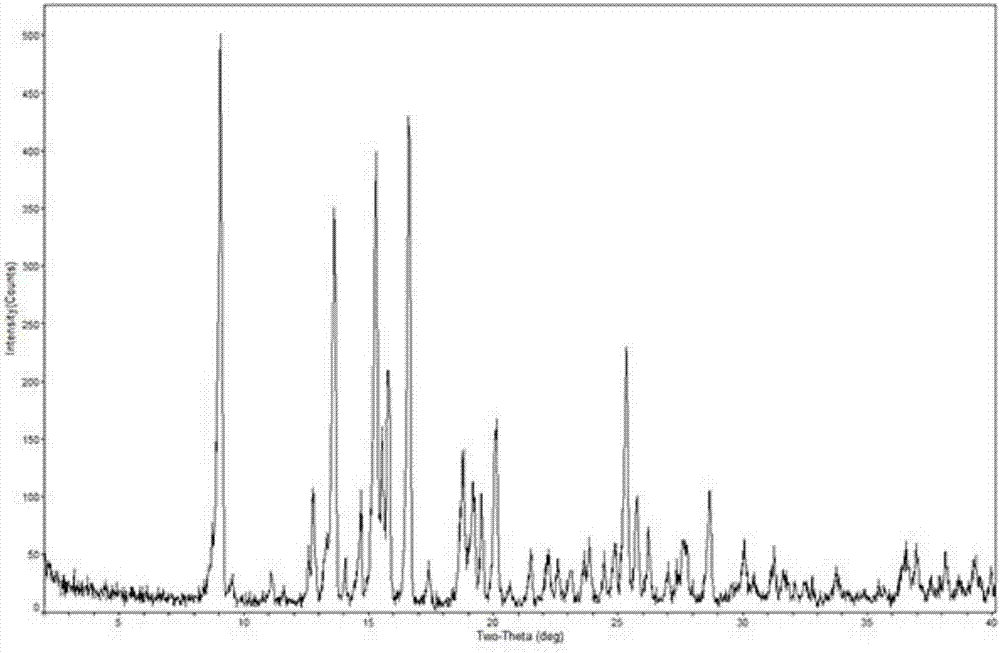
[0039] The X-ray powder diffraction is determined for the dried crystal, and its X-ray powder diffraction is measured at 2θ=8.1°, 9.8°, 12.0°, 14.6°, 15.0°, 16.4°, 16.7°, 17.3°, 17.9°, 19.7° , There are characteristic peaks at 24.8°, as detailed in the attached instructions image 3 shown. The TG-DTA spectrogram of the prepared mometasone furoate crystalline form M is as detailed in the attached description. Figure 4 shown.
[0040] Take an appropriate amount of mometasone furoate sample, put it in a weighing bottle, spread it into a thin layer with a thickness of ≤5 mm, and carry out the following experiments. The results are shown in Table 1. The content is detected a...
Embodiment 2
[0051] Invention Example 2 Comparative Study on Stability and Preparation Quality of Mometasone Furoate Suspension Nasal Spray
[0052] Table 2 Prescription of mometasone furoate suspension nasal spray
[0053]
[0054] Refer to commercially available mometasone furoate nasal spray Prescription, prepared mometasone furoate crystal form M suspension nasal spray (group A), mometasone furoate monohydrate suspension nasal spray (group B) and mometasone furoate anhydrous crystal form FORM1 suspension Type nasal spray (Group C), and compared for stability. Group A used micronized mometasone furoate crystal form M, and the particle size was D (0.99) = 3.789 μm when detected by a laser particle size analyzer. Group B used micronized mometasone furoate monohydrate, and the particle size was D(0.99)=3.758 μm when detected by laser particle size analyzer. Group C used micronized mometasone furoate anhydrous FORM1, and the particle size was D(0.99)=3.685 μm when detected by laser p...
Embodiment 3
[0067] Invention Example 3 Preparation and Stability Investigation of Mometasone Furoate Crystal Form M Suspension Nasal Spray
[0068] Table 5 Suspension Nasal Spray Prescription
[0069]
[0070]
[0071] The main ingredient contained in Examples 3-1 to 3-10 is mometasone furoate crystal form M, and the dosage is 0.5 g. The preparation method refers to Example 2.
[0072] 3.1 Stability
[0073] Take 10 bottles for each group, store them for 24 months at 30°C±2°C, 60%RH±5%RH relative humidity, and measure the contents at 0, 12 months and 24 months respectively (using mometasone furoate The measured results are shown in Table 6 below; with reference to the particle size determination method in the USP fluticasone propionate nasal spray standard, the particle size of mometasone furoate was detected at 0 o'clock and at the end of 24 months, and the results of the particle size of mometasone furoate are shown in Table 7 below:
[0074]Table 6 Mometasone furoate content in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com