Polymorph of axially chiral enantiomer of URAT1 inhibitor
A technology of polymorphism and axial chirality, applied in the field of polymorph I and its application in the field of medicine, can solve the problems of difficult drug preparation and storage, thermodynamic instability, poor fluidity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
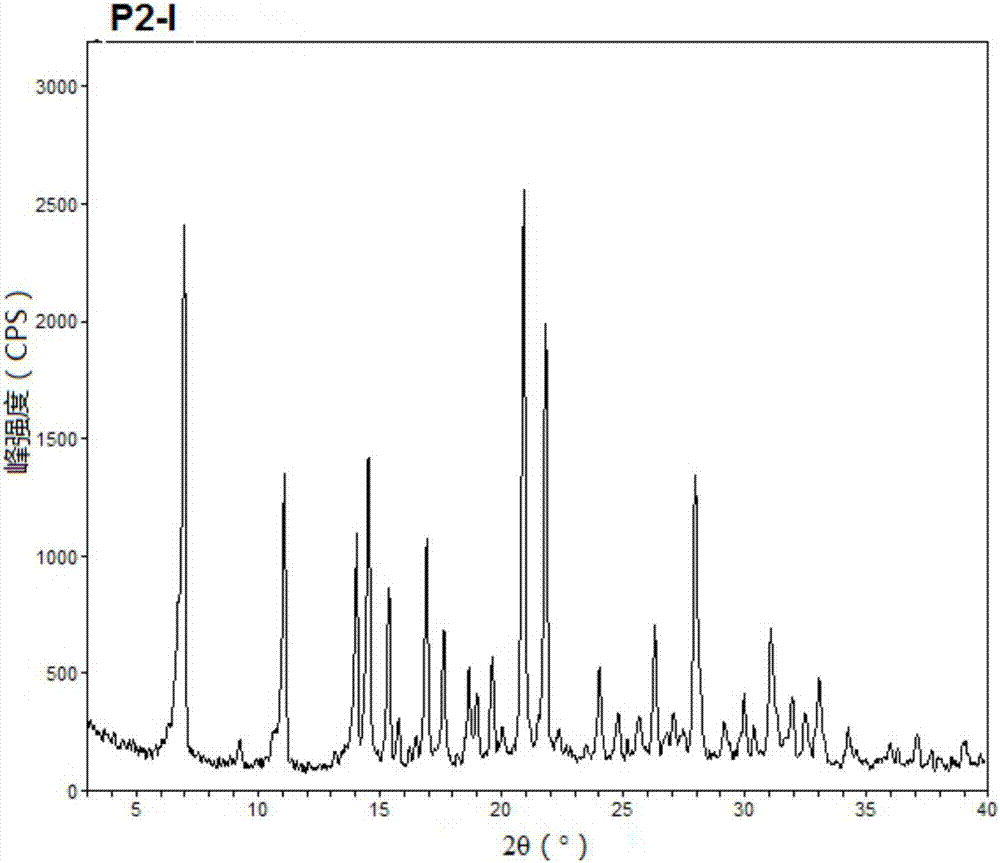
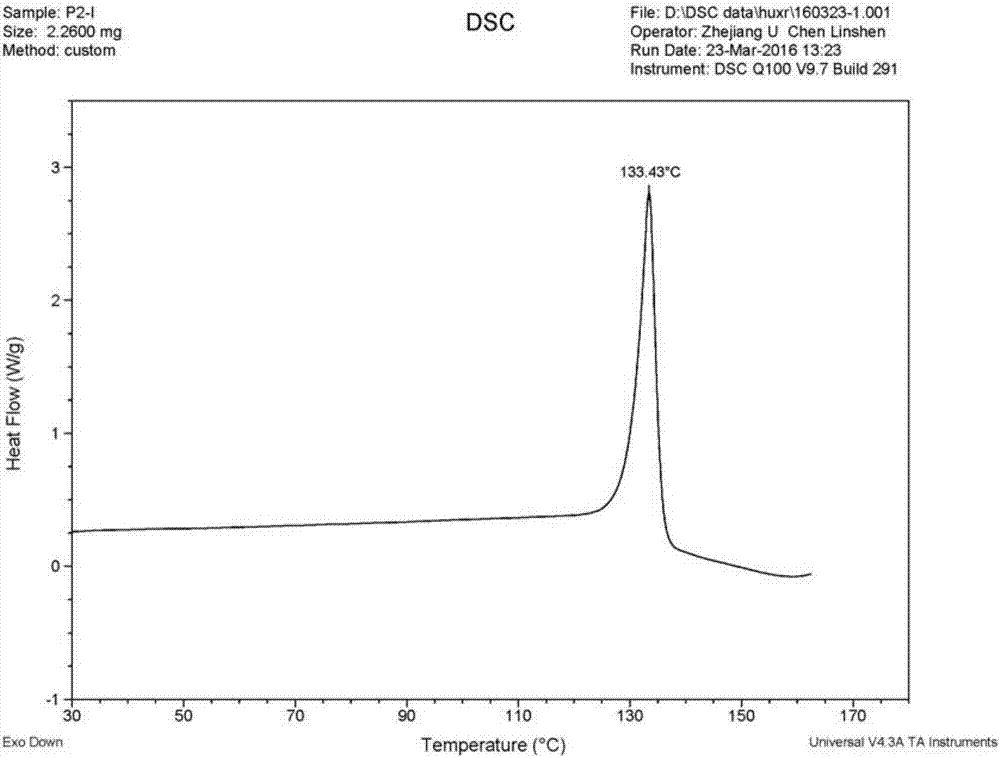
[0076] The preparation of embodiment 1 polymorph I
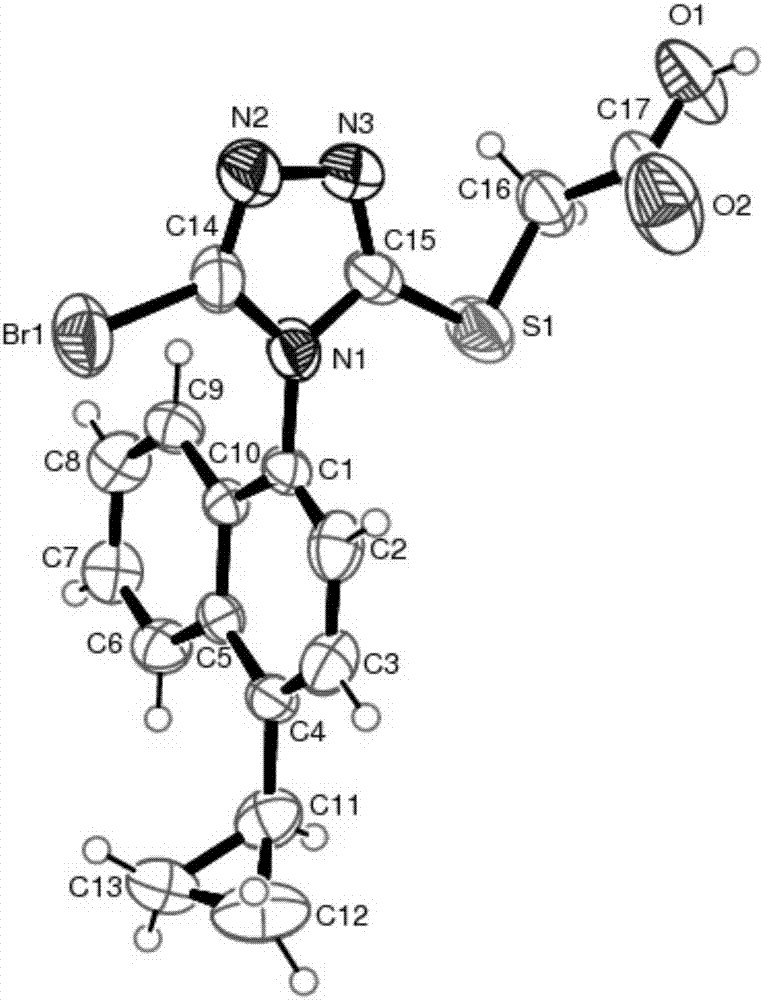
[0077] Take 0.2g sample of amorphous (S)-2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid , added to 40mL cyclohexane, heated to 50°C to dissolve for 2 hours to make a saturated solution, stirred and cooled to 10°C for crystallization, filtered, and baked at 45°C for 2 hours to obtain 0.15g of (S)-2-(5- A sample of polymorph I of bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid.
Embodiment 2
[0078] The preparation of embodiment 2 polymorph I
[0079] Take 0.2g sample of amorphous (S)-2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid , added to 50mL of heptane, heated and dissolved at 60°C for 2 hours to make a saturated solution, stirred and cooled to 10°C for crystallization, filtered, and baked at 45°C for 2 hours to obtain 0.13g of (S)-2-(5-bromo- A sample of polymorph I of 4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid.
Embodiment 3
[0080] The preparation of embodiment 3 polymorph I
[0081] Take 0.2g sample of amorphous (S)-2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid , added to 50mL of n-hexane, heated and dissolved at 60°C for 2 hours to make a saturated solution, stirred and cooled to 10°C for crystallization, filtered, and baked at 45°C for 2 hours to obtain 0.17g of (S)-2-(5-bromo- A sample of polymorph I of 4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com