Mn/Co-based low-temperature SCO catalyst and preparation method thereof
A catalyst, low temperature technology, used in physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems of limited industrial application, poor stability, etc., and achieve good low-temperature denitrification. Activity, avoidance of agglomeration, the effect of structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) 520 μL of 50% Mn(NO 3 ) 2 solution and 1.2gCo(NO 3 ) 3 ·6H 2 O (the molar ratio of manganese and cobalt is 1:1) was added to 50 mL of DMF, 1000 μL of formic acid and 10 μL of water were added, and the solution was fully mixed by magnetic stirring for 30 min to obtain a mixed solution;
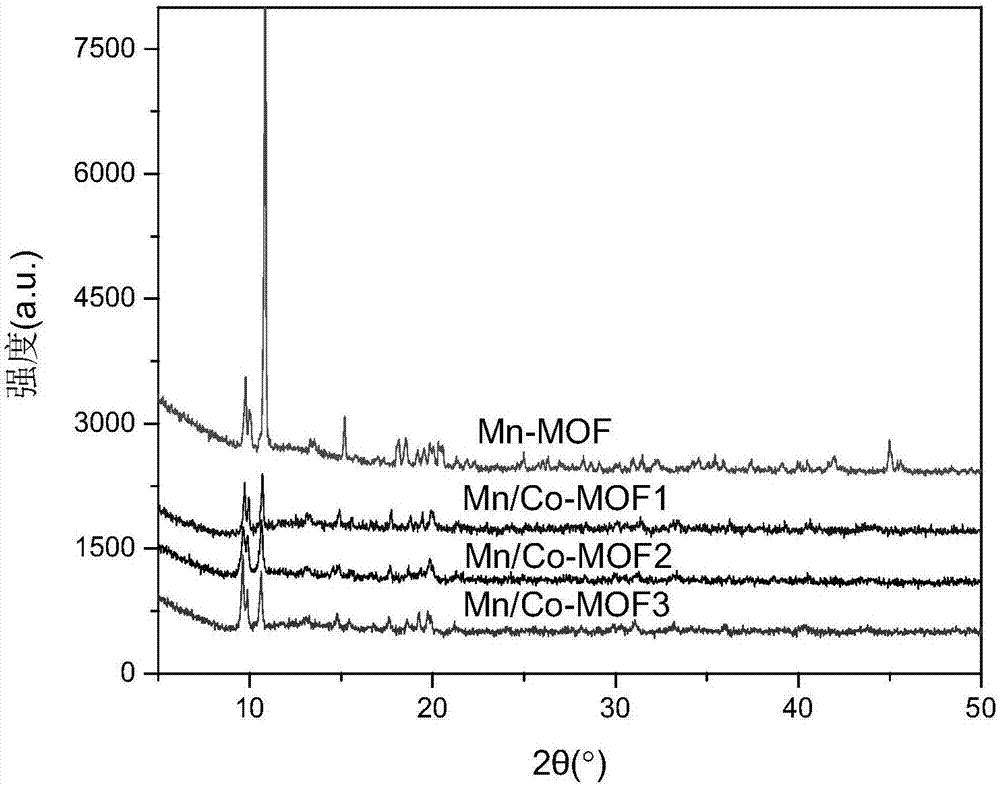
[0038] (2) Place the mixed solution in a drying oven at 80°C and react for 20 hours. After the solution is cooled, remove the supernatant, add fresh DMF, soak and activate at room temperature for 12 hours, and obtain an activated crystal material (Mn / Co-MOF1);
[0039] (3) The activated crystal material is impregnated with DMF, washed and purified, filtered, and the filtered solid material is placed in a vacuum oven at 50°C for drying;
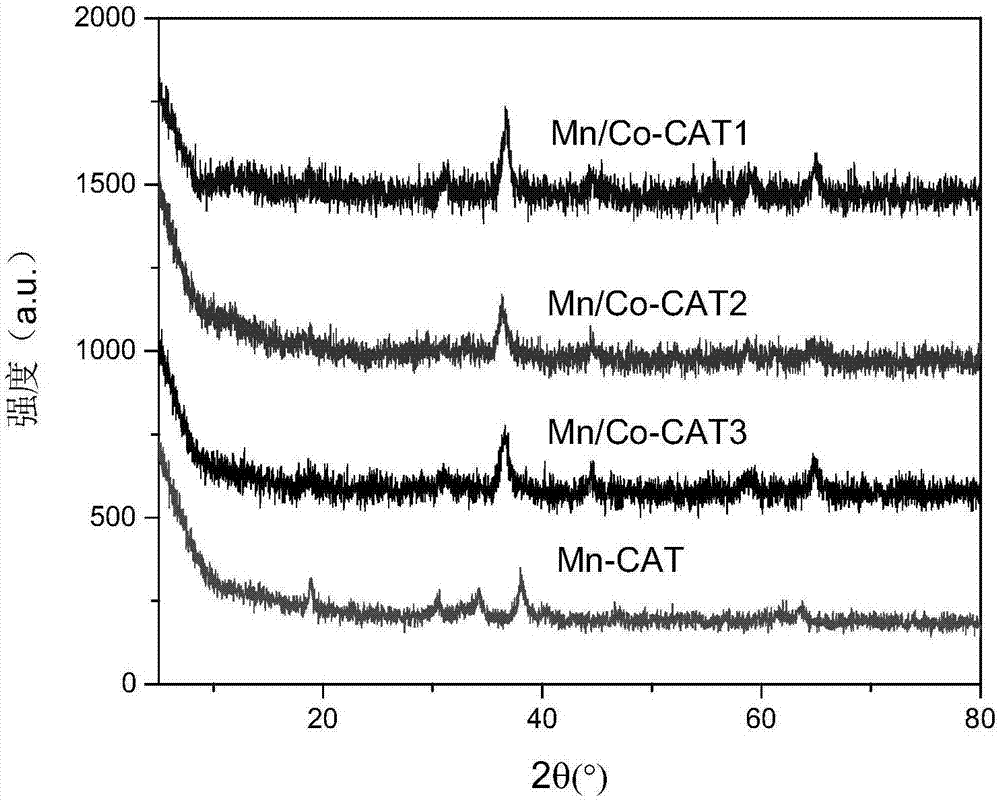
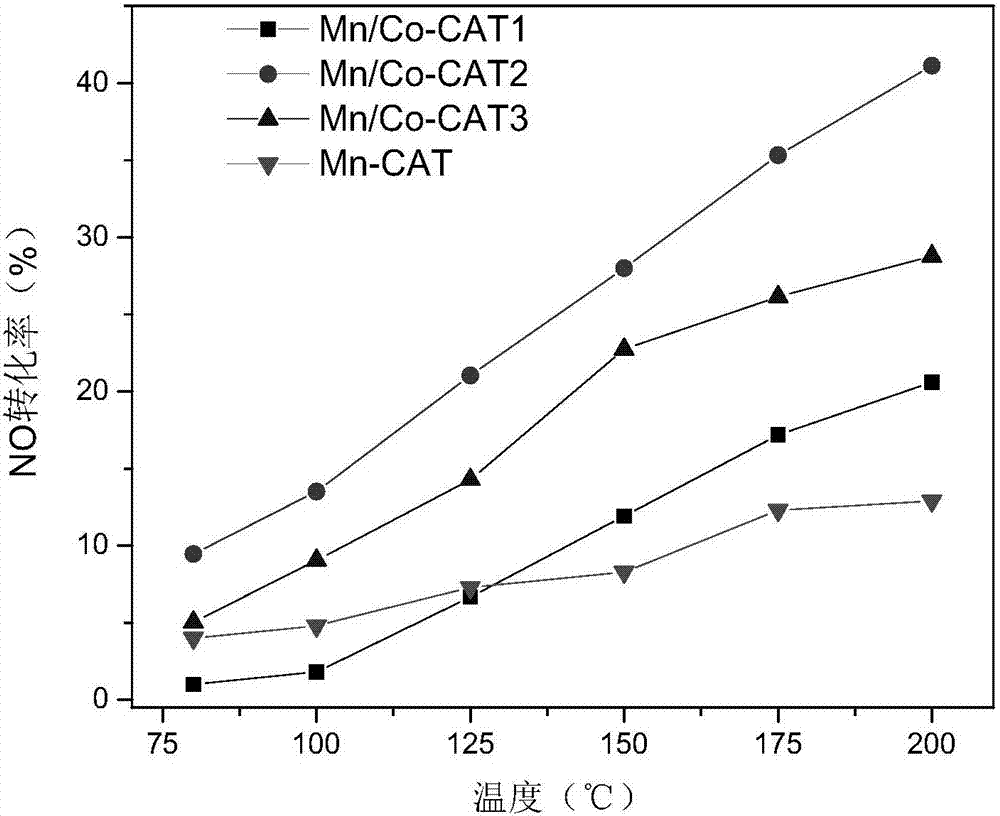
[0040] (4) The dried solid material was put into a muffle furnace and calcined at 300 °C for 2 h to prepare a Mn-based low-temperature SCO catalyst (denoted as Mn / Co-CAT1).
Embodiment 2
[0042] (1) 520 μL of 50% Mn(NO 3 ) 2 solution and 0.64gCo(NO 3 ) 3 ·6H 2 O (the molar ratio of manganese and cobalt is 1:0.5) was added to 50 mL of DMF, 1200 μL of formic acid and 30 μL of water were added, and the solution was fully mixed by magnetic stirring for 30 min to obtain a mixed solution;
[0043] (2) Place the mixed solution in a drying oven at 100°C and react for 24 hours. After the solution is cooled, remove the supernatant, add fresh DMF, soak and activate at room temperature for 18 hours, and obtain an activated crystal material (Mn / Co-MOF2);
[0044] (3) The activated crystalline material is impregnated with DMF, washed and purified, filtered, and the solid material obtained after filtering is placed in a vacuum drying oven at 80° C. for drying;
[0045] (4) The dried solid material was put into a muffle furnace and calcined at 350° C. for 2.5 h to prepare a Mn-based low-temperature SCO catalyst (denoted as Mn / Co-CAT2).
Embodiment 3
[0047] (1) 520 μL of 50% Mn(NO 3 ) 2 solution and 2.40gCo(NO 3 ) 3 ·6H 2 O (the molar ratio of manganese and cobalt is 1:2) was added to 50 mL of DMF, 1500 μL of formic acid and 50 μL of water were added, and the solution was fully mixed by magnetic stirring for 30 min to obtain a mixed solution;
[0048] (2) Place the mixed solution in a drying oven at 120°C and react for 30 hours. After the solution is cooled, remove the supernatant, add fresh DMF, soak and activate at room temperature for 24 hours, and obtain an activated crystal material (Mn / Co-MOF3);
[0049] (3) The activated crystal material is impregnated with DMF, washed and purified, filtered, and the filtered solid material is dried in a vacuum oven at 100°C;
[0050] (4) The dried solid material was put into a muffle furnace and calcined at 400°C for 3.0 h to prepare a Mn-based low-temperature SCO catalyst (denoted as Mn / Co-CAT3).
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com