Synthetic method of 4-isothiocyanato-2-(trifluoromethyl) benzonitrile
A technology of trifluoromethyl and isothiocyanate, which is applied in the field of synthesis of pharmaceutical intermediates to achieve the effect of high product yield and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
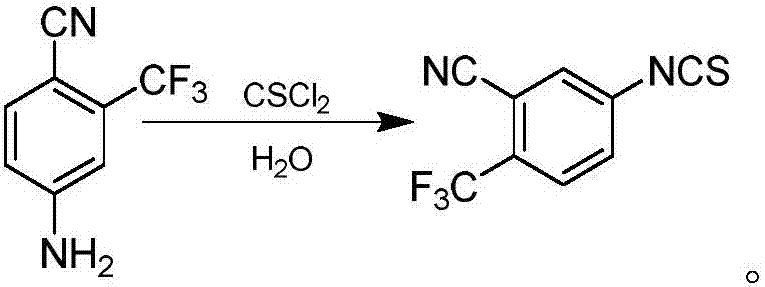
[0029] The synthetic method of 4-isothiocyanate-2-(trifluoromethyl) benzonitrile comprises the following steps:
[0030] a. Dissolve 3-trifluoromethyl-4-cyanoaniline (Compound I) (1.12g, 6mmol, 1.0eq) and phenyl thiochloroformate (1.04g, 6mmol, 1.0eq) in 20mL of dichloromethane , Stirring was started at about 25°C, and the reaction was complete after 2 hours. After filtration, the insoluble matter was removed, the filtrate was collected, and the solvent was removed under reduced pressure to obtain a yellow solid (II).
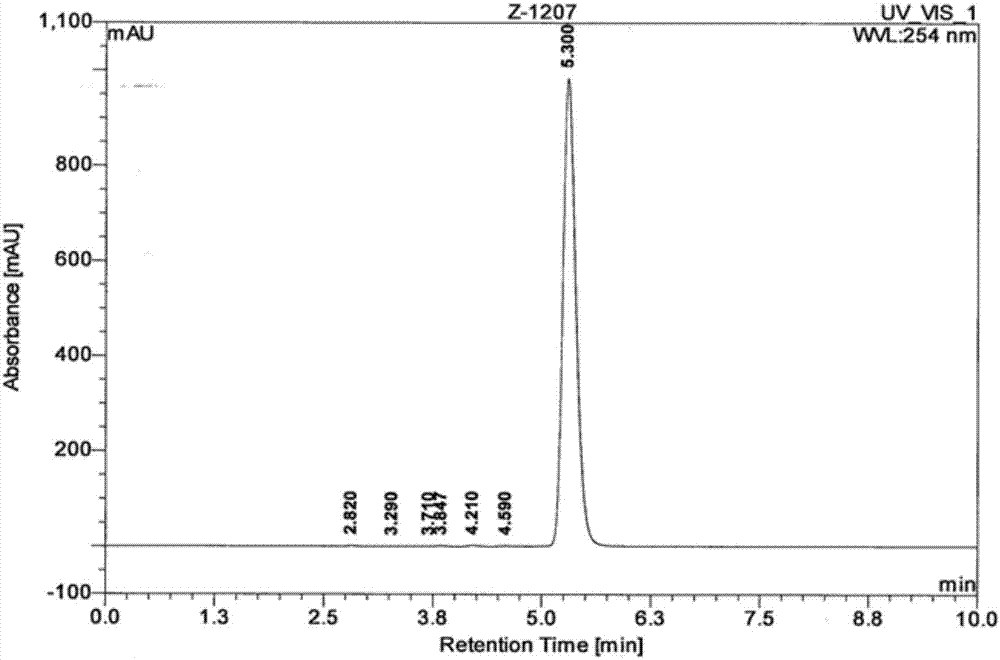
[0031] b. Weigh the yellow solid (II) (0.64 g, 2 mmol), dissolve it in 20 mL of toluene, heat up and stir to reflux, and keep for 18 h. The insoluble matter in the reaction solution was filtered off, the filtrate was collected, and concentrated under reduced pressure to obtain a crude product. After column chromatography, 274 mg of the target product 4-isothiocyanate-2-(trifluoromethyl)benzonitrile was obtained, with a yield of 60.1%.
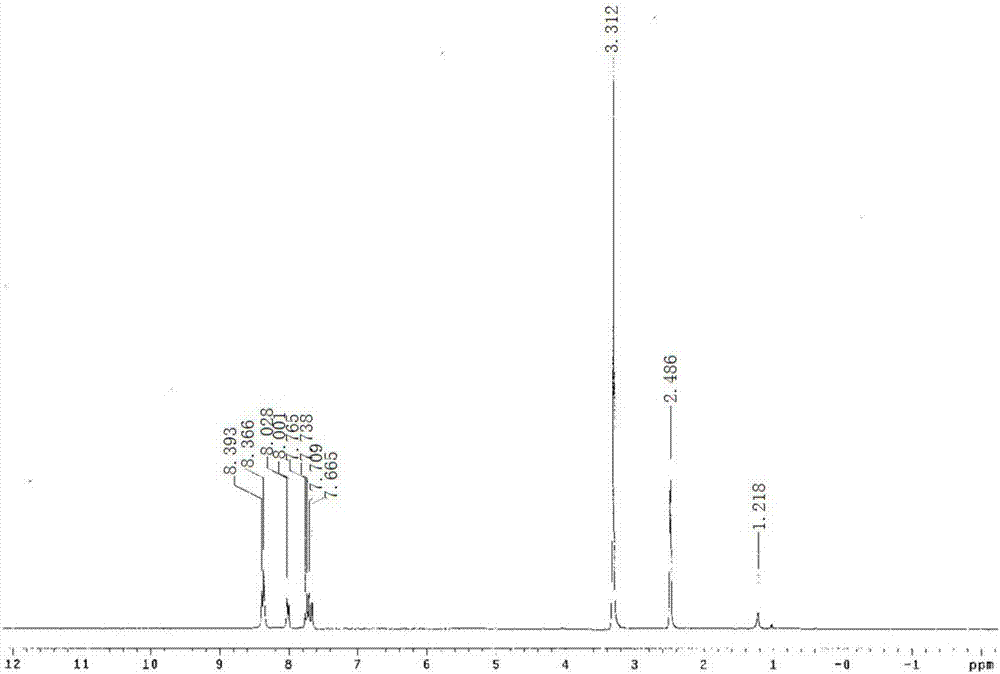
[0032] Target product 4...
Embodiment 2
[0036] The synthetic method of 4-isothiocyanate-2-(trifluoromethyl) benzonitrile comprises the following steps:
[0037] a. Dissolve 3-trifluoromethyl-4-cyanoaniline (Compound I) (1.12g, 6mmol, 1.0eq) and phenyl thiochloroformate (0.69g, 4mmol, 0.67eq) in 20mL of dichloromethane , Stirring was started at about 25°C, and the reaction was complete after 2 hours. After filtration, the insoluble matter was removed, the filtrate was collected, and the solvent was removed under reduced pressure to obtain a yellow solid (II).
[0038] b. Weigh the yellow solid (II) (0.64 g, 2 mmol), dissolve it in 20 mL of toluene, heat up and stir to reflux, and keep for 18 h. The insoluble matter in the reaction solution was filtered off, the filtrate was collected, and concentrated under reduced pressure to obtain a crude product. After column chromatography, 330 mg of the target product 4-isothiocyanate-2-(trifluoromethyl)benzonitrile was obtained, with a yield of 72.3%.
[0039] The target prod...
Embodiment 3
[0043] The synthetic method of 4-isothiocyanate-2-(trifluoromethyl) benzonitrile comprises the following steps:
[0044] a. Dissolve 3-trifluoromethyl-4-cyanoaniline (Compound I) (1.12g, 6mmol, 1.0eq) and phenyl thiochloroformate (0.52g, 3mmol, 0.52eq) in 20mL of dichloromethane , Stirring was started at about 25°C, the reaction was complete after 2 hours, filtered to remove insoluble matter, the filtrate was collected, and the solvent was removed under reduced pressure to obtain a yellow solid (II).
[0045] b. Weigh the yellow solid (II) (0.64 g, 2 mmol), dissolve it in 20 mL of toluene, heat up and stir to reflux, and keep for 18 h. The insoluble matter in the reaction solution was filtered off, the filtrate was collected, and concentrated under reduced pressure to obtain a crude product. After column chromatography, 361 mg of the target product 4-isothiocyanate-2-(trifluoromethyl)benzonitrile was obtained, with a yield of 79.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com