Synthetic method of N-methylpiperidine
A technology of methyl piperidine and a synthesis method, which is applied in the synthesis field of N-methyl piperidine, can solve the problems of serious pollution, complicated operation, large energy consumption and the like, and achieves a reaction with less impurities, simple operation and low energy consumption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
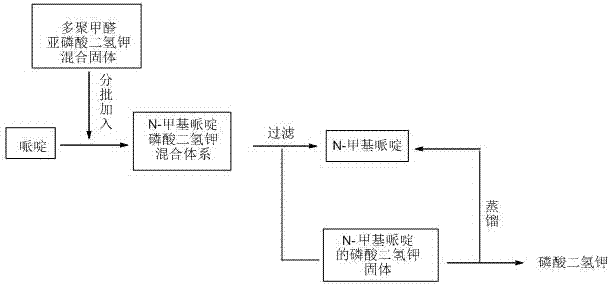
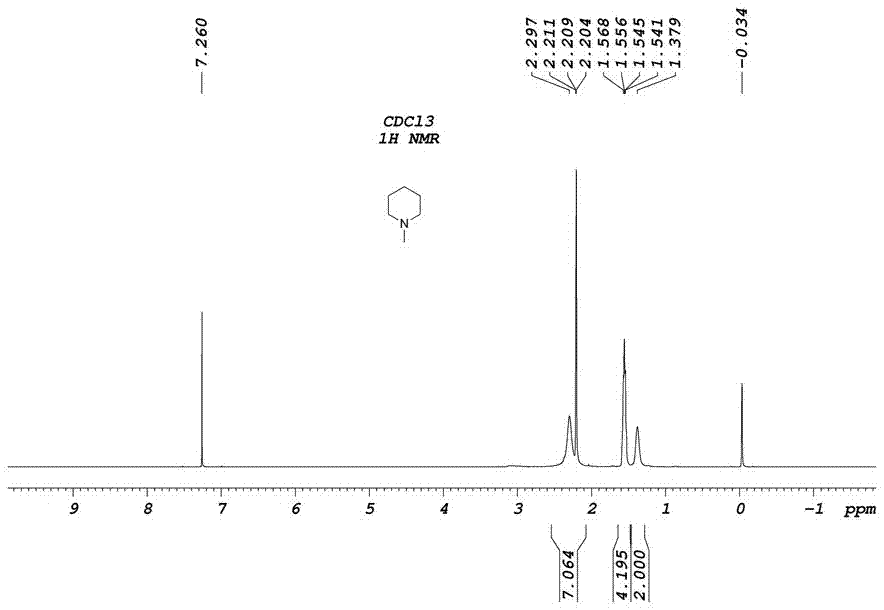
[0026] Weigh 170g of piperidine in a 500mL three-neck flask with mechanical stirring, heat it to 80°C in an oil bath, weigh 72g of paraformaldehyde and 144g of potassium dihydrogen phosphite, mix well, and then add them to piperidine in batches , the total addition time is controlled within 1h, divided into 4 batches, and the interval between each batch is 15 minutes. During the addition process, attention should be paid to prevent flushing; after the addition is completed, react for 2 hours. After the piperidine reaction is complete by thin-layer chromatography, cool to room temperature. 130g of filtrate and residue were obtained by suction filtration, and the residue obtained by suction filtration was placed in a reaction flask and heated to 110°C for distillation until no liquid was distilled out. The remaining solid after distillation was potassium dihydrogen phosphate 128g, which was recovered and used as fertilizer, Liquid is 54g, and the composition of filtrate and disti...
Embodiment 2
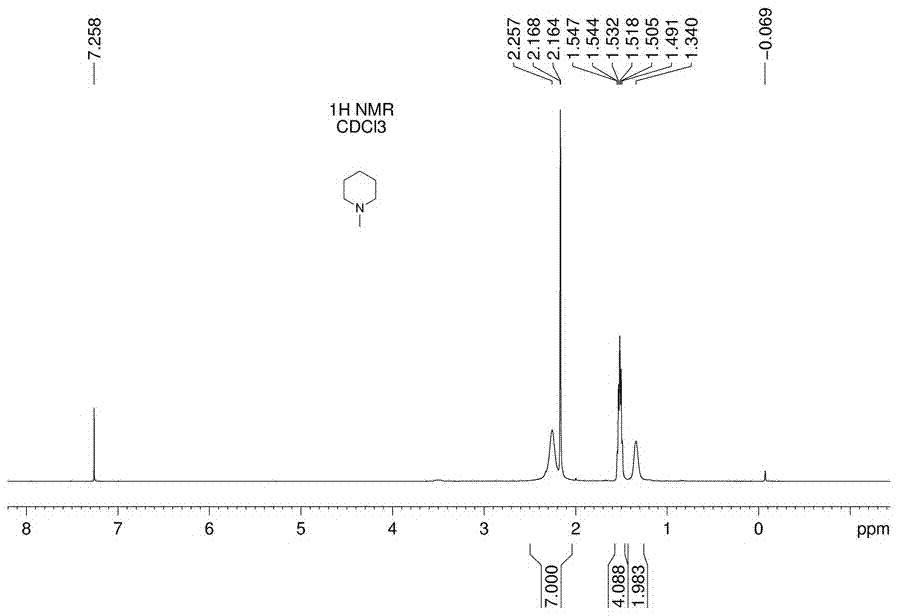
[0028] Weigh 170g of piperidine in a 500mL three-necked flask with mechanical stirring, heat it to 90°C in an oil bath, weigh 72g of paraformaldehyde and 144g of potassium dihydrogen phosphite, mix well, and then add them to piperidine in batches , the total addition time is controlled within 1.5h, divided into 6 batches in total, and the interval between each batch is 15 minutes. During the addition, attention should be paid to prevent flushing; after the addition is completed, the reaction is 1.5h. After the piperidine reaction is complete by thin-layer chromatography, it is cooled to At room temperature, 140g of filtrate and residue were obtained by suction filtration; the residue obtained by suction filtration was placed in a reaction flask and heated to 115°C for distillation until no liquid was distilled out. The remaining solid after distillation was potassium dihydrogen phosphate 126g, which was recovered and used as a fertilizer. Distillate is 46g, and the composition ...
Embodiment 3
[0030] Weigh 170g of piperidine in a 500mL three-neck flask with mechanical stirring, heat it in an oil bath to 100°C, weigh 72g of paraformaldehyde and 144g of potassium dihydrogen phosphite, mix well, and then add them to piperidine in batches , the total addition time is controlled within 50 minutes, divided into 5 batches, and the interval between each batch of addition is 10 minutes. During the addition process, attention should be paid to prevent flushing; after the addition is completed, the reaction is 2.5h. After the piperidine reaction is complete by thin-layer chromatography, it is cooled to At 60°C, 152g of filtrate and residue were obtained by suction filtration; the residue obtained by suction filtration was placed in a reaction flask and heated to 120°C for distillation until no liquid was distilled out. The remaining solid after distillation was potassium dihydrogen phosphate 122g, which was recovered and used as fertilizer , distillate is 36g, and the compositi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com