Use of isoquinoline derivatives for diabetic wound healing
A technology for wound healing and diabetes, applied in the field of isoquinoline derivatives, can solve the problems of difficult-to-prevent complications and no reported effects of isoquinoline derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0041] 1. have the preparation of the compound of formula I of the present invention
[0042] Dopamine (1.6 g), 10 mL of methanol, 1 mL of 1N hydrochloric acid and 2 mL of 99% acetaldehyde were sequentially added into a 50 mL round bottom flask and stirred at room temperature for 6 hours. The concentrate obtained by concentration under reduced pressure was loaded onto a Lobar RP-18 column (size B, Merck) and eluted with 0.05% aqueous formic acid to provide pure norhyolinine (1.0 g) for 1H NMR.
[0043] Using ESI-TOF mass spectrometer and NMR spectrum analysis, the characteristic data of norhyrogynine are as follows:
[0044] 1H NMR (CD 3 OD,400MHz)δ6.63(1H,s),6.57(1H,s),4.30(1H,q,J=6.8Hz,H-1),3.40(1H,dt,J=12.6,5.6Hz,H a -3),3.20(1H,ddd,J=12.6,8.2,5.6Hz,H b -3),2.92(1H,ddd,J=16.8,8.2,5.8Hz,H a -4),2.80(1H,dt,J=16.8,5.6Hz,H b -4), 1.55 (3H, d, J=6.8Hz, Me-1); ESIMS: m / z 180 ([M+H] + ).
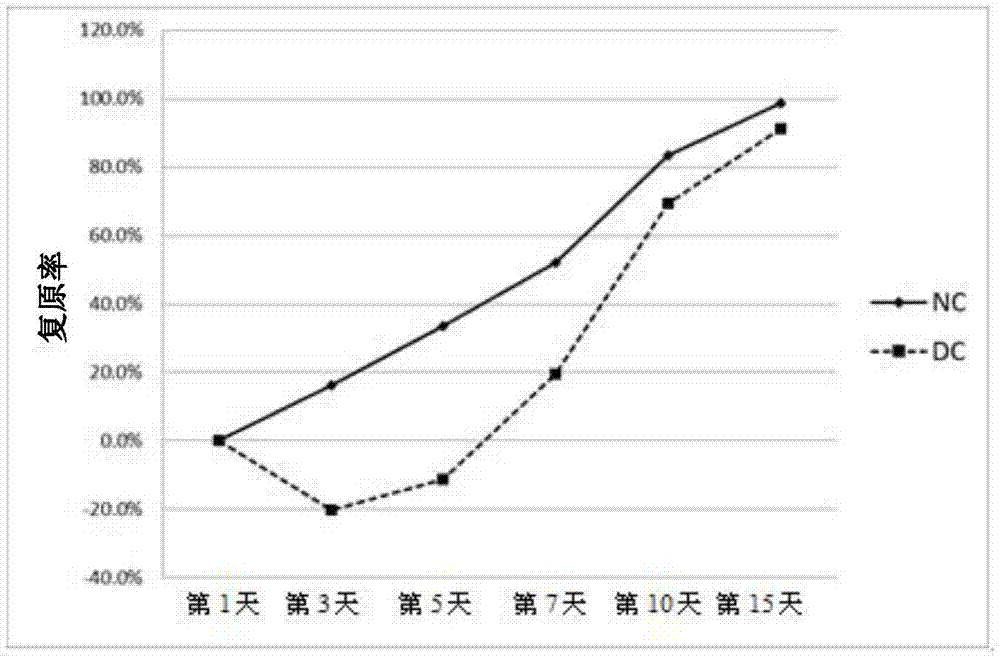
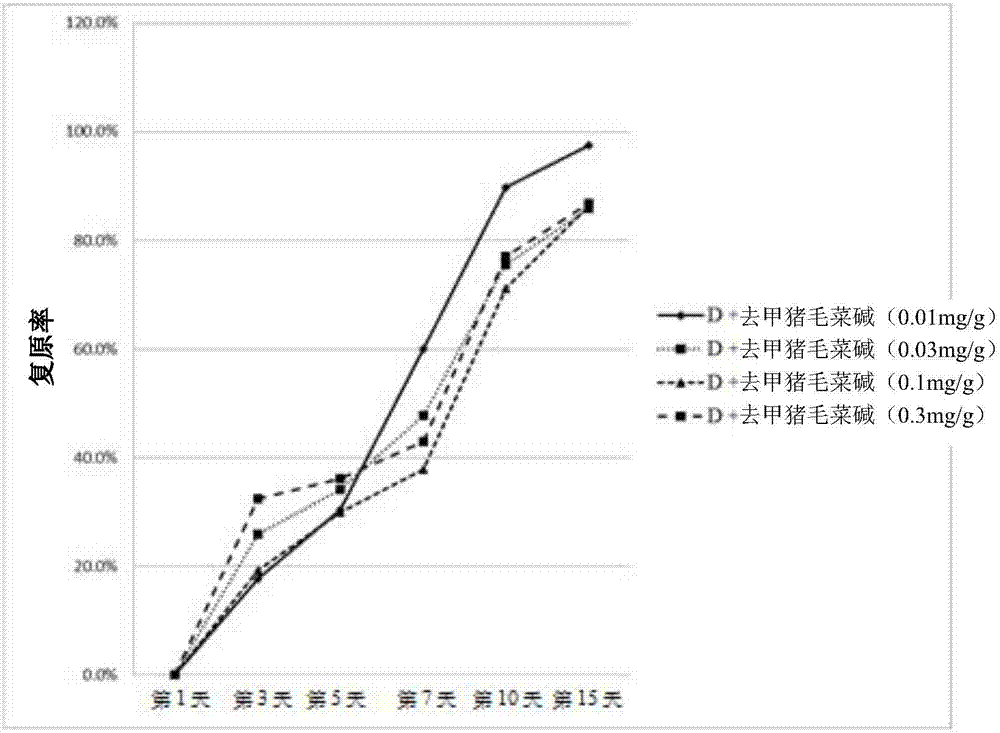
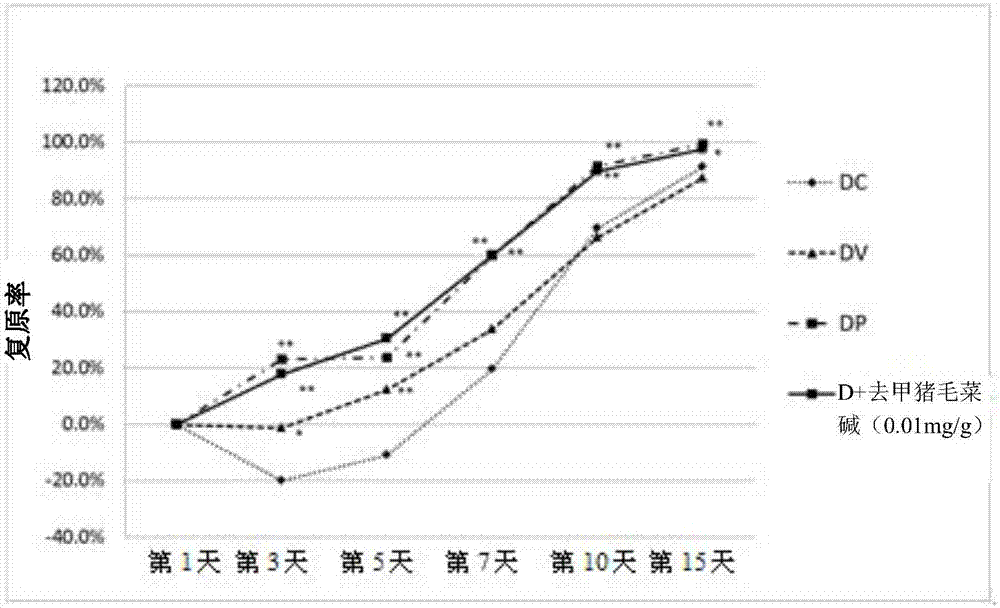
[0045] 2. Evaluation of the effect of norhyrogynine in the treatment of diabetic woun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com