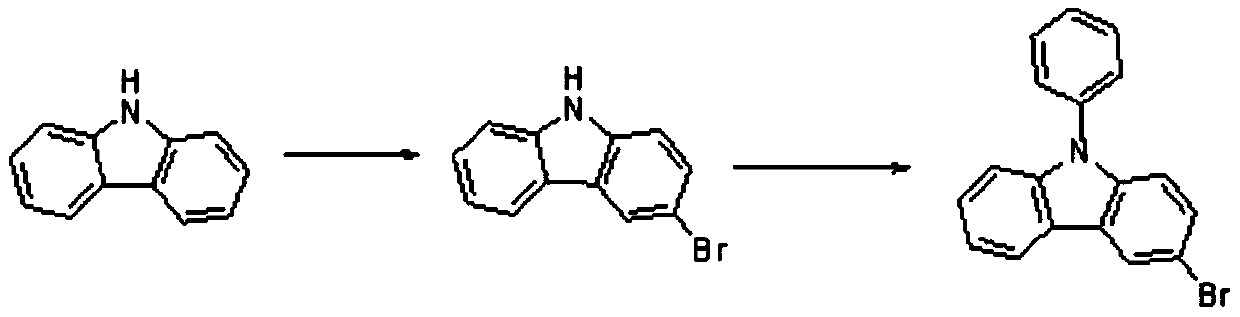
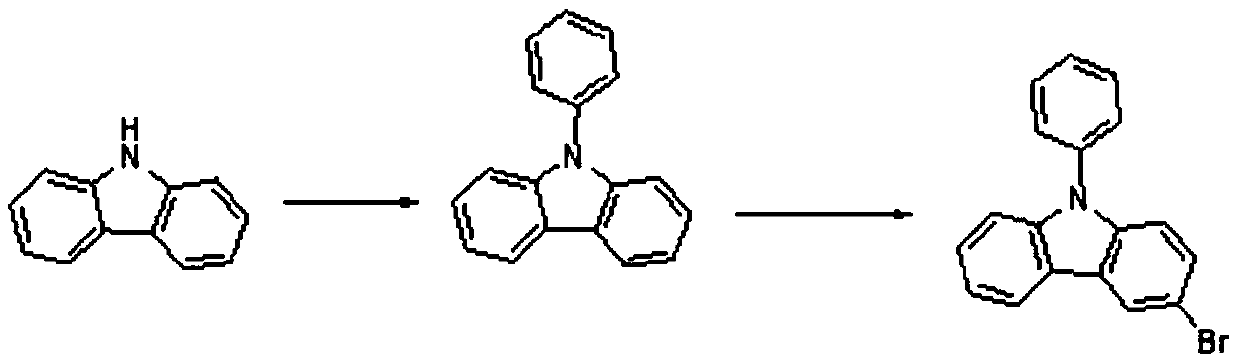
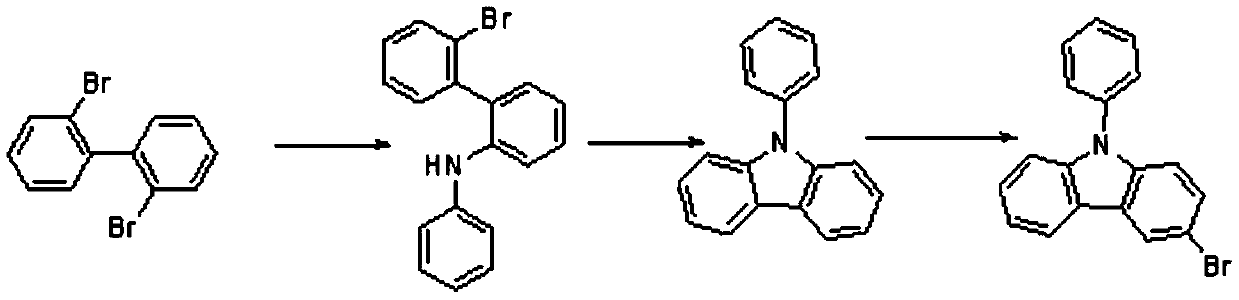
A kind of preparation method of high-purity 3-bromo-9-phenylcarbazole
A technology of phenylcarbazole and aminophenyl, which is applied in the field of preparation of high-purity 3-bromo-9-phenylcarbazole, can solve problems such as difficulty in large-scale production, high cost of raw materials, and harsh reaction conditions, and achieves The effect of avoiding the process of removing dibromine, reducing production costs, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] This embodiment provides a preparation method of 3-bromo-9-phenylcarbazole, specifically, the method includes the following steps:
[0061] (1) Add 23.59 g of p-dibromobenzene (molecular weight 235.9, 0.1 mol) and 235.9 mL of dichloromethane into a reaction flask equipped with stirring, condenser and thermometer, control the temperature at 30°C, and add concentrated Nitric acid 9.26g (concentration: 68%) (molecular weight: 63, 0.1mol), the reaction was completed after two hours of reaction. Wash the obtained organic phase with water to neutrality, add 10 g of sodium sulfate to dry, and then concentrate to dryness under reduced pressure to obtain 27.52 g of 1,4-dibromo-2-nitrobenzene (molecular weight: 280.9, 0.098 mol); MS (FAB): m / z 280(M+)
[0062] (2) 1,4-dibromo-2-nitrobenzene, 16.58 g of diphenylamine (molecular weight 169.2, 0.098 mol), 0.7 g of cuprous oxide (molecular weight 143.08, 0.0049 mol), 18 - Crown ether-6 1.29g (molecular weight 264.32, 0.0049mol), po...
Embodiment 2
[0068] This embodiment provides a preparation method of 3-bromo-9-phenylcarbazole, specifically, the method includes the following steps:
[0069] (1) Add 23.59 g of p-dibromobenzene (molecular weight 235.9, 0.1 mol) and 353.8 mL of dichloroethane into a reaction flask equipped with stirring, condenser, and thermometer, control the temperature at 25°C, and add concentrated Nitric acid 10.19g (68% content) (molecular weight 63, 0.11mol), the reaction was completed after two hours of reaction. The obtained organic phase was washed with water until neutral, dried by adding 10 g of sodium sulfate, and then concentrated to dryness under reduced pressure to obtain 27.66 g of 1,4-dibromo-2-nitrobenzene (molecular weight: 280.9, 0.098 mol).
[0070] (2) 1,4-dibromo-2-nitrobenzene prepared in step (1), 16.58g of diphenylamine (molecular weight 169.2, 0.098mol), 0.485g of cuprous chloride (molecular weight 98.99, 0.0049mol), 1.29g of 18-crown-6 (molecular weight 264.32, 0.0049mol), 27....
Embodiment 3
[0075] This embodiment provides a preparation method of 3-bromo-9-phenylcarbazole, specifically, the method includes the following steps:
[0076] (1) Add 23.59 g of p-dibromobenzene (molecular weight 235.9, 0.1 mol) and 471.8 mL of chloroform into a reaction flask with stirring, condenser and thermometer, control the temperature at 25 °C, and add concentrated nitric acid 11.1 l g (68% content) (molecular weight 63, 0.12 mol), the reaction ended after two hours of reaction. The obtained organic phase was washed with water until neutral, dried by adding 10 g of sodium sulfate, and then concentrated to dryness under reduced pressure to obtain 27.41 g of 1,4-dibromo-2-nitrobenzene (molecular weight: 280.9, 0.097 mol).
[0077] (2) 1,4-dibromo-2-nitrobenzene, 16.41 g of diphenylamine (molecular weight 169.2, 0.097 mol), 0.69 g of cuprous bromide (molecular weight 143.35, 0.00485 mol), Add 1.28g of 18-crown-6 (molecular weight 264.32, 0.00485mol), cesium carbonate 1.58g (molecular...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com