A kind of dmxaa-pyranoxanthone hybrid compound and its preparation method and application
A technology of compounds and hybrids, applied in the field of preparation of anti-tumor drugs, can solve the problems of anti-tumor cell proliferation activity not particularly active, patients restless, visual impairment, etc., to achieve inhibition of in vitro proliferation, rapid response, and reduced toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of D-P hybrid compounds.
[0043] 1.1 Preparation of D-P-3 hybrid compound
[0044] Including the following 7 steps:
[0045] 1.1.1 Preparation of 2,5-dibromo-3,4-dimethylbenzoic acid
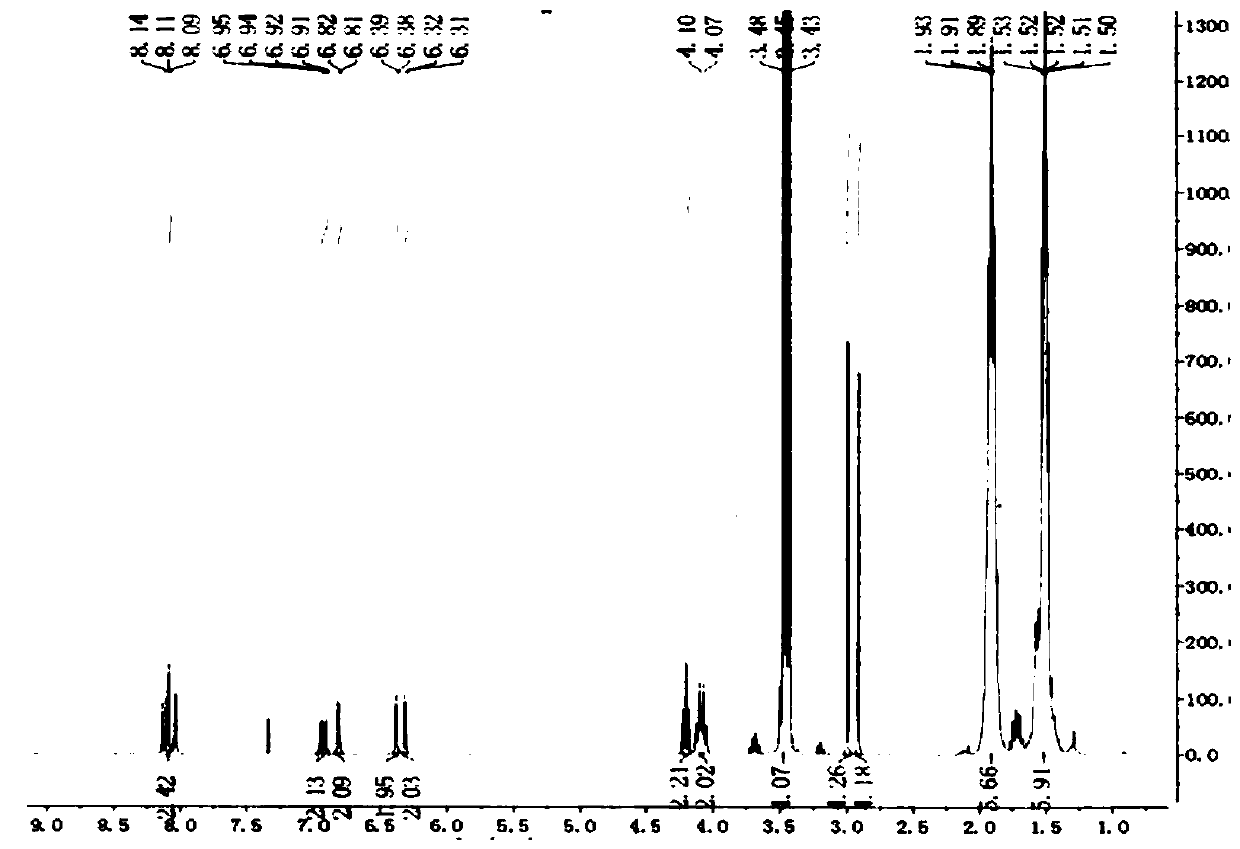
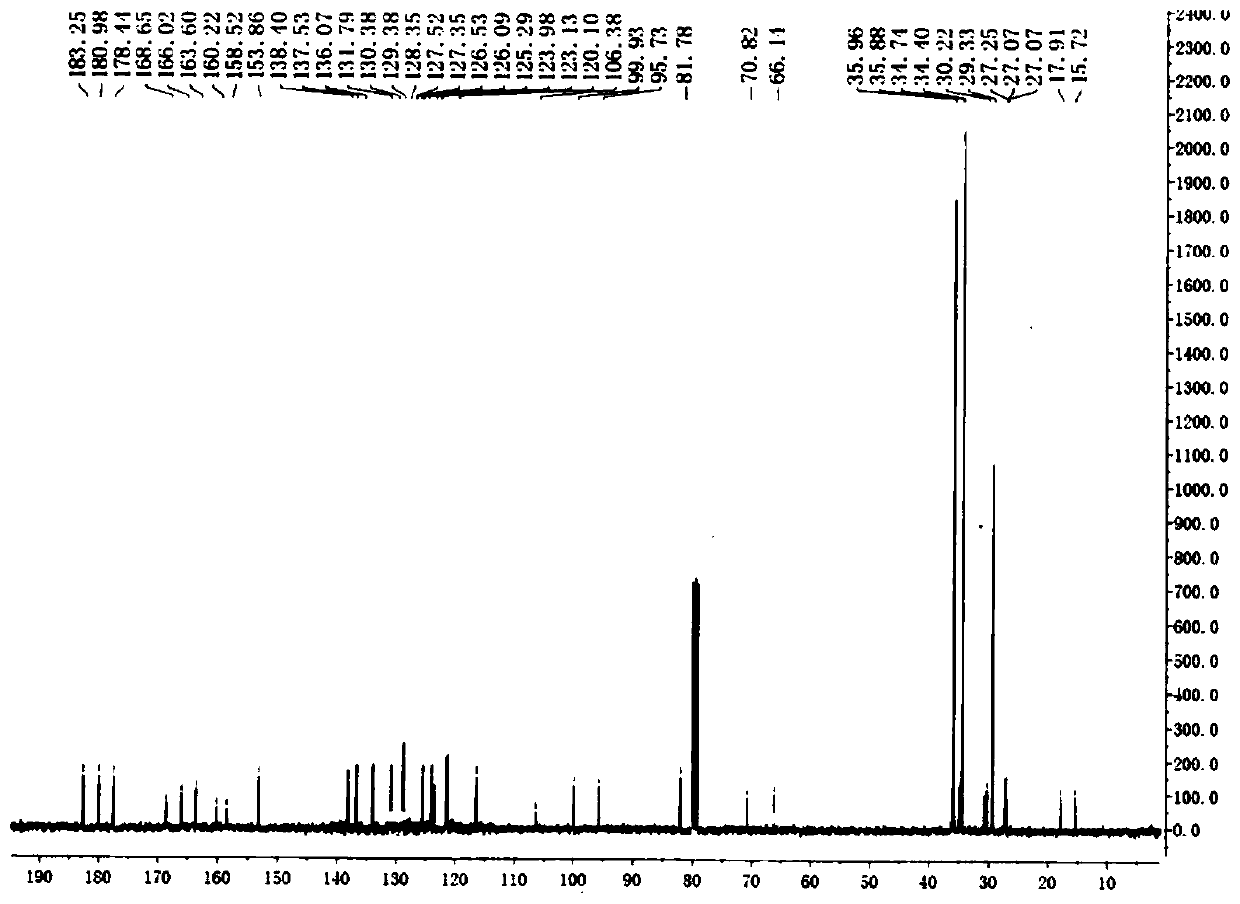
[0046] Dissolve 3,4-dimethylbenzoic acid (0.164g, 1.0mmol) in 20mL of concentrated sulfuric acid, add NBS (0.212g, 1.2mmol) in batches, and stir at room temperature for 12h; then slowly pour the reaction mixture into ice-water solution , there was a white precipitate, which was collected by filtration and washed with cold water; the crude product was recrystallized and purified with distilled water to obtain 0.303 g of white crystals, with a yield of 98.0%, M.p.: 131-134°C. 1 H NMR (400MHz, DMSO-d 6 )δ:7.68(s,1H),2.48(s,3H),2.46(s,3H); 13 CNMR (75MHz, CDCl 3 )δ: 177.1, 136.5, 135.4, 135.1, 133.7, 127.0, 123.3, 19.3, 18.9; ESI-MS (m / z): 307.9 [M+H] + . The above data confirmed that the compound was 2,5-dibromo-3,4-dimethylbenzoic acid.
[0047] 1.1.2 Preparation of 2-(...
Embodiment 2
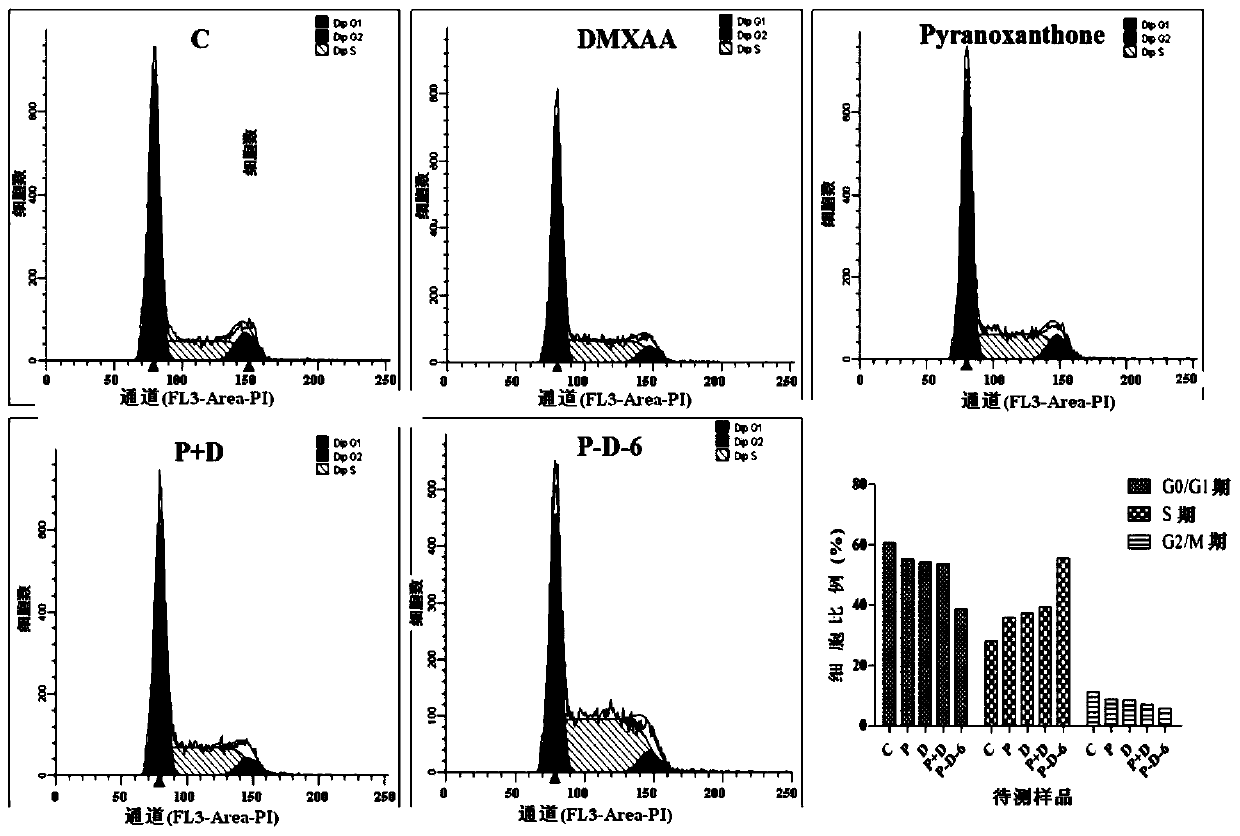
[0082] D-P-3~D-P-6 and D / P Combined Drug Inhibitory Activity of Tumor Cell Proliferation in Vitro
[0083] Experimental method: Cells in the logarithmic growth phase were inoculated in a 96-well plate at a seeding density of 1×10 4 cells / well, cultured overnight. When the degree of fusion reaches more than 70%, drug treatment is added, and a blank group, a control group, and different concentration administration groups are set in the experiment. The D-P-n hybrid compound was serially diluted starting from 200 μM, and three replicate wells were set up for each group. After incubation for 24 h, add 10 μL of MTT solution to each well and place at 37 °C with 5% CO 2 Continue in the incubator, protect from light when MTT solution. After incubation for 4 hours, discard the solution in each well, be careful not to suck away the crystals, and finally add 100 μL of fresh DMSO solution to each well, shake it on a shaker for 15 minutes in the dark, and place it on a microplate reader...
Embodiment 3
[0093] Toxicity of D-P-6 to normal cells
[0094] Experimental method is the same as embodiment 1.
[0095] The results indicated that the compound D-P-6 showed a lower inhibitory effect on cell proliferation in vitro on the human liver cell line HL-7702 and the mouse embryonic fibroblast cell line NIH / 3T3.
[0096] Specifically, the IC of compound D-P-6 after acting on HL-7702 cells for 24 and 48 hours 50 is 452.29±41.20, 351.98±31.26μM; IC of compound D-P-6 after acting on NIH / 3T3 cells for 24 and 48h 50 are 378.03±37.22, 293.22±25.34 μM. It shows that the compound D-P-6 has strong cytotoxicity to the tested tumor cell lines, but less toxic to normal cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com