Application of novel thiazole derivative in treatment of virus infection
A virus infection, virus technology, applied in the application field of the treatment of virus infection, can solve the problems of long research and development cycle and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] (E)-4-(2-Chlorophenyl)-2-[1-methyl-2-(2-carboxybenzylidene)hydrazino]thiazole 1
[0098]
[0099] 2-Methylthiosemicarbazide (1-1)
[0100] Weigh 2.5g (17.3mmol) of methylhydrazine sulfate in a 250ml single-necked bottle, add 100ml of ethanol, add 1.6g (20.8mmol) of ammonium thiocyanate under stirring, heat to reflux, and react for 72 hours, then cool the reaction solution to After suction filtration at room temperature, the filtrate was spin-dried by silica gel column chromatography (DCM / MeOH=40:1), and the second by-product was separated to obtain 0.63 g of a white powdery solid, with a yield of 34.2%. 1HNMR(400MHz,DMSO-d6,ppm)δ7.36(s,2H),4.89(s,2H),3.41(s,3H).GC-MS(EI)calcd forC2H7N3S[M]+105.0,found 105.0.
[0101] 2-Methyl-1-(2-carboxybenzyl)thiosemicarbazide (1-2)
[0102] Weigh 80mg (0.76mmol) of compound (1) in a 50ml single-necked bottle, add 20ml of ethanol, add o-carboxybenzaldehyde 114mg (0.76mmol) under stirring, heat to reflux, monitor the reaction by T...
Embodiment 2
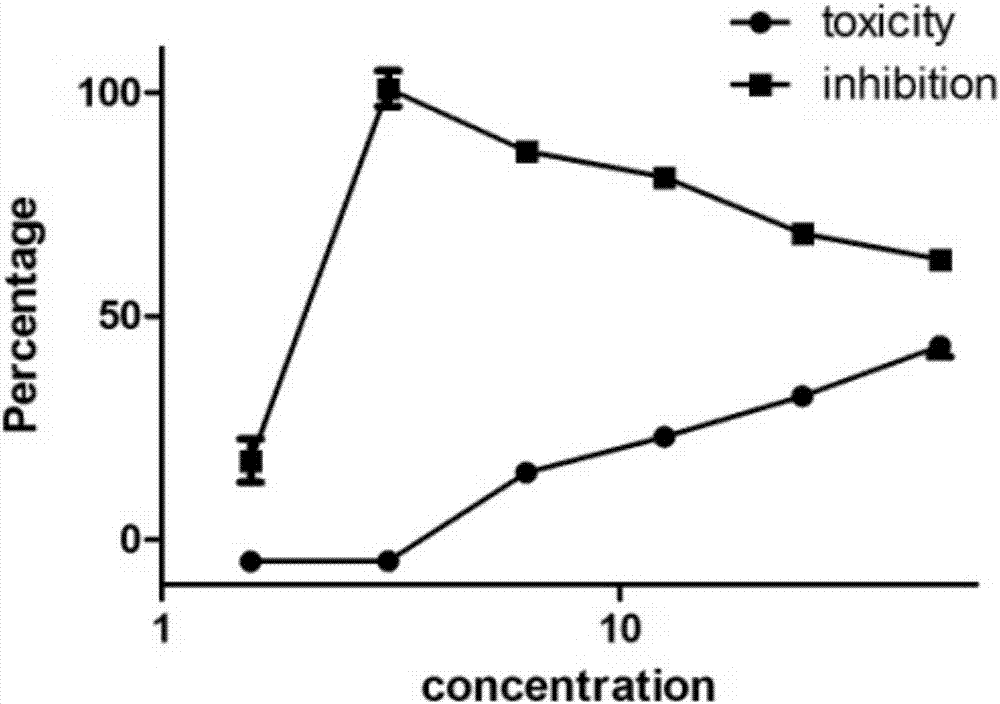
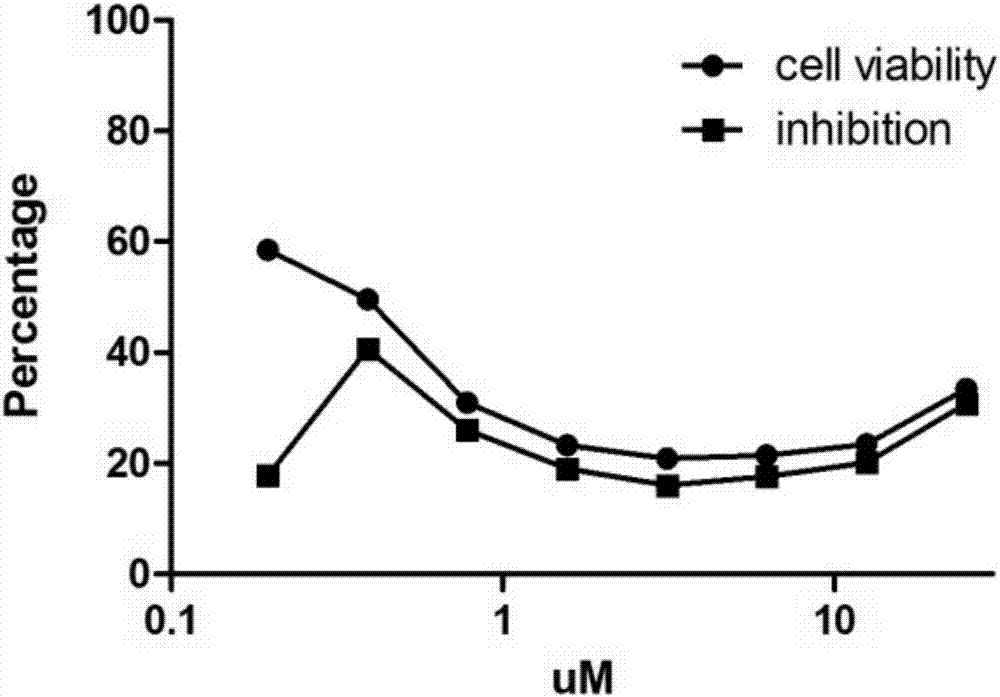
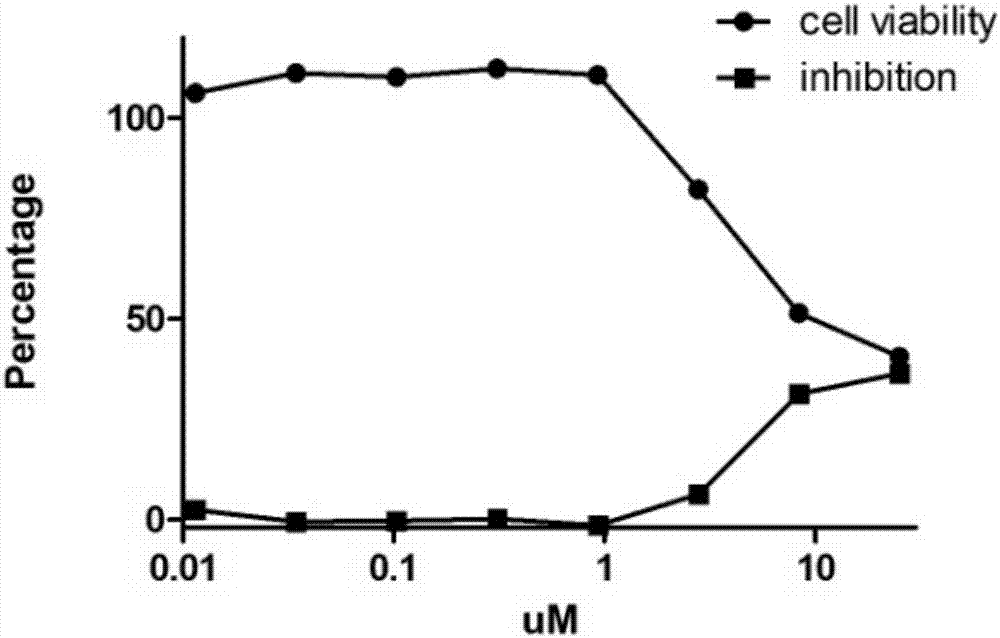
[0193] Embodiment 2 (activity evaluation)
[0194] Activity test for inhibiting virus replication at the cellular level
[0195] Determination of drug IC50 (half inhibitory concentration): MDCK cells or Vero cells or RD cells were plated on a 96-well plate and grown to more than 90% after 12 hours of culture for use. Drugs were serially diluted 2 times (1×10 -1 to 1×10 -10 ). The monolayer of MDCK cells was sucked off the culture medium, washed once with PBS, 50 μl of drug solution of corresponding dilution was added to each well, and 50 μl of virus solution containing 100 times TCID50 was added to each well, and four replicate wells were made for each dilution. 37°C, 5% CO2After culturing for 3-5 days, observe the generation of CPE (cytopathic), record the number of positive wells that can protect cells from producing CPE and negative wells that cannot protect cells from producing CPE in four duplicate wells, and find out The drug dilution factor that can inhibit half of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com