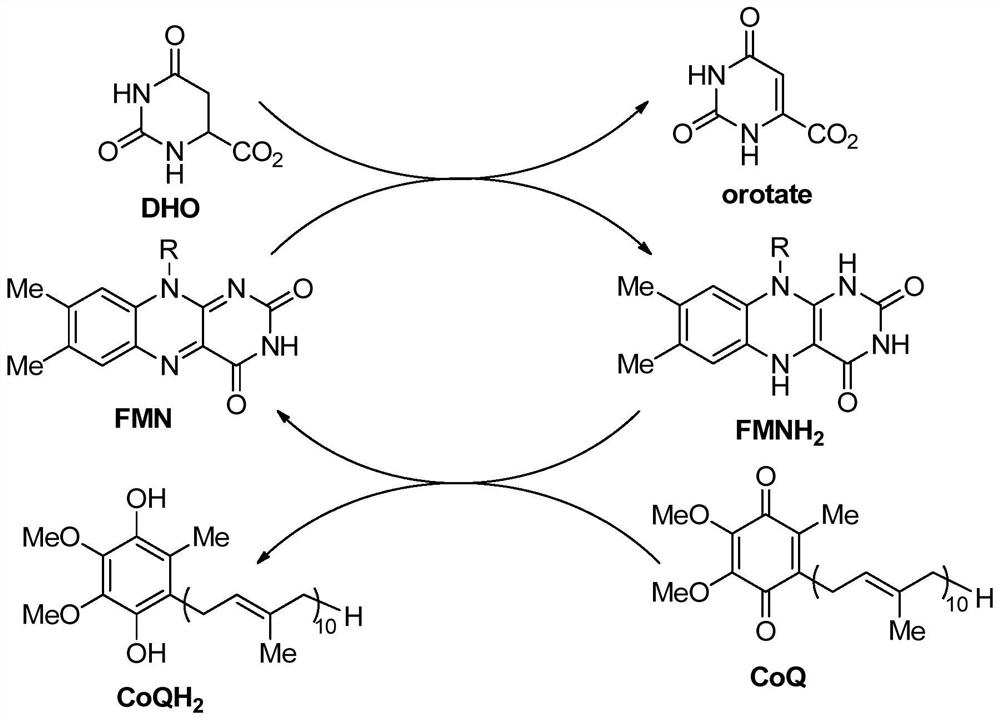
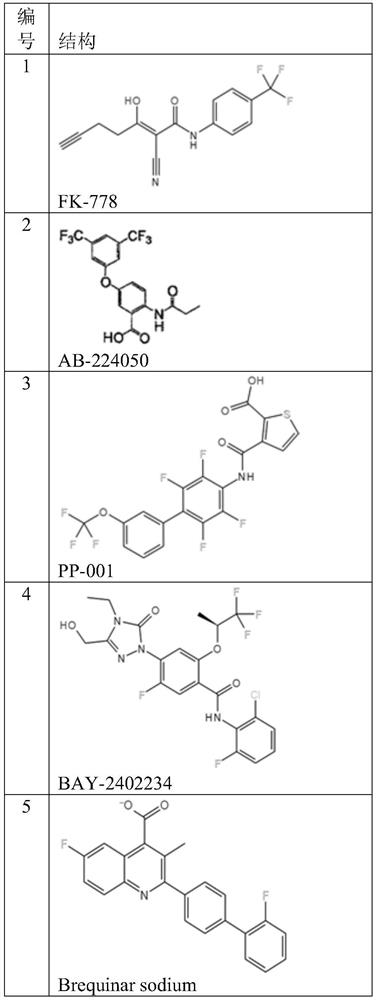
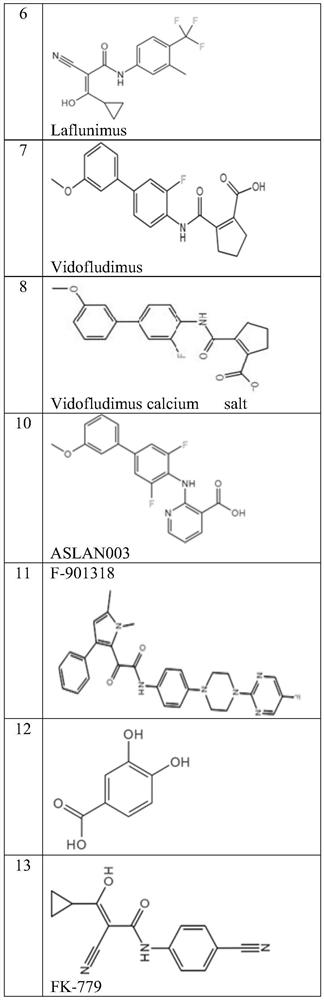
DHODH inhibitor of anti-RNA virus drug and application of DHODH inhibitor
A technology of RNA virus and DHODH, which can be applied in the direction of antiviral agents, resistance to vector-borne diseases, medical preparations containing active ingredients, etc., and can solve problems such as less work and less effort
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1. The inhibitory activity and cytotoxicity evaluation of the compound of the present invention to 2019 new coronavirus (2019-nCoV), Ebola virus, avian influenza virus A / GuangZhou / 99 (H9N2)
[0063] Materials and methods: Buquina is commercially available with a purity of over 98%, and DHODH inhibitors are purchased from compound libraries or reagent companies.
[0064] Detection methods and results:
[0065] 1. Anti-2019 novel coronavirus (2019-nCoV) drug efficacy experiment detected by fluorescent quantitative PCR method:
[0066]References (Wu Zhong, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Research (2020), 1–3, Zhou, P., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin.Nature 2020) method, infected with 2019BetaCoV / Wuhan / WIV04 / 2019 strain) infection amount MOI=0.03 on Vero E6 cells (ATCC-1586), and added different dilutions of drugs at the s...
Embodiment 2
[0087] Example 2 Inhibitory activity of compounds of the present invention to H3N2, H1N1, H7N9
[0088] Materials and methods: DHODH inhibitors were purchased from compound libraries or reagent companies.
[0089] Detection method and results: Activity test for inhibiting virus replication at the cellular level
[0090] Determination of drug IC50 (half inhibitory concentration): MDCK cells or Vero cells or RD cells were plated on a 96-well plate, cultured for 12 hours and grown to more than 90% for later use. Drugs were serially diluted 2-fold (1×10-1 to 1×10-10). The monolayer of MDCK cells was sucked off the culture medium, washed once with PBS, 50 μl of drug solution of corresponding dilution was added to each well, and 50 μl of virus solution containing 100 times TCID50 was added to each well, and four replicate wells were made for each dilution. 37°C, 5% CO 2After culturing for 3-5 days, observe the production of CPE (cytopathic), record the number of positive wells th...
Embodiment 3
[0103] Embodiment 3. The antiviral activity evaluation of the combination of the compound of the present invention and other antiviral drugs
[0104] The inventors further tested some higher active DHODH inhibitors and other antiviral drugs of the prior art, including lopinavir, ritonavir, ribavirin, remdesivir, oseltamivir, One or more combinations of Tamiflu, Laninamivir, Peramivir and Chloroquine (Chloroquine Phosphate).
[0105] It was found that some highly active DHODH inhibitors combined with these antiviral drugs can produce better therapeutic effects; Chloroquine phosphate) combined treatment effect is relatively better.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com