Materials and methds to enhance hematopoietic stem cells engraftment procedures
A cell and blood stem technology, applied in biochemical equipment and methods, blood/immune system cells, fusion cells, etc., can solve problems such as limited results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
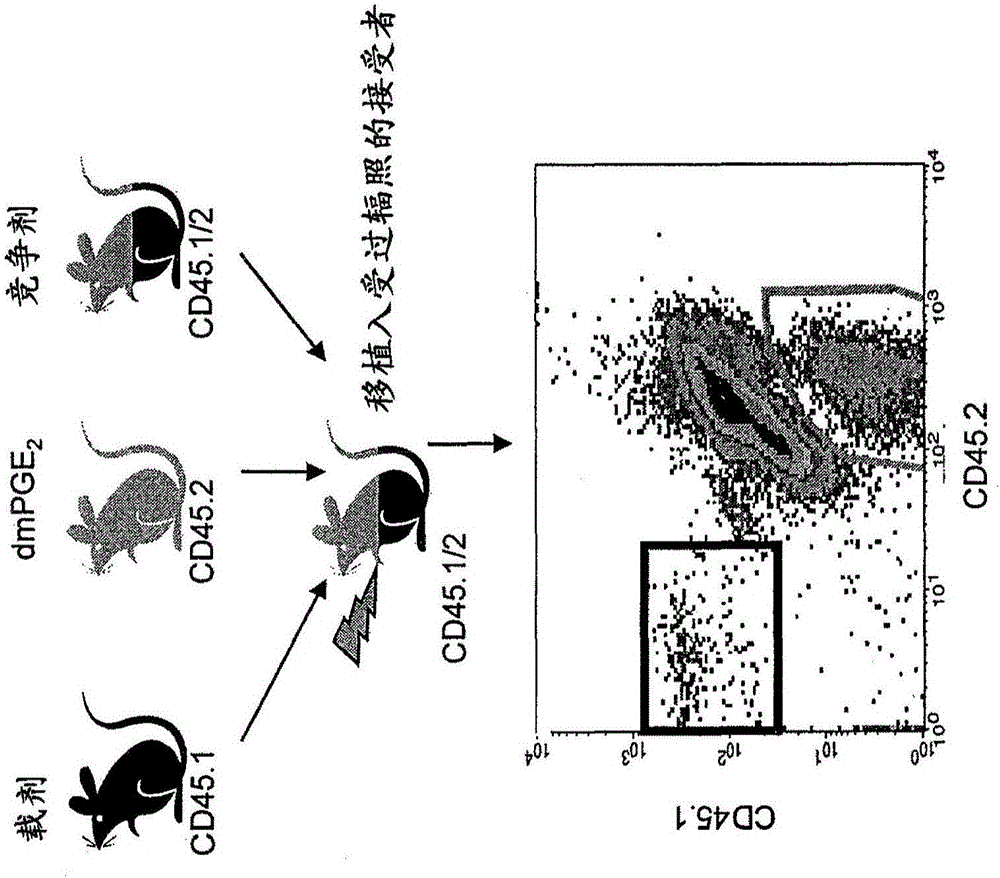
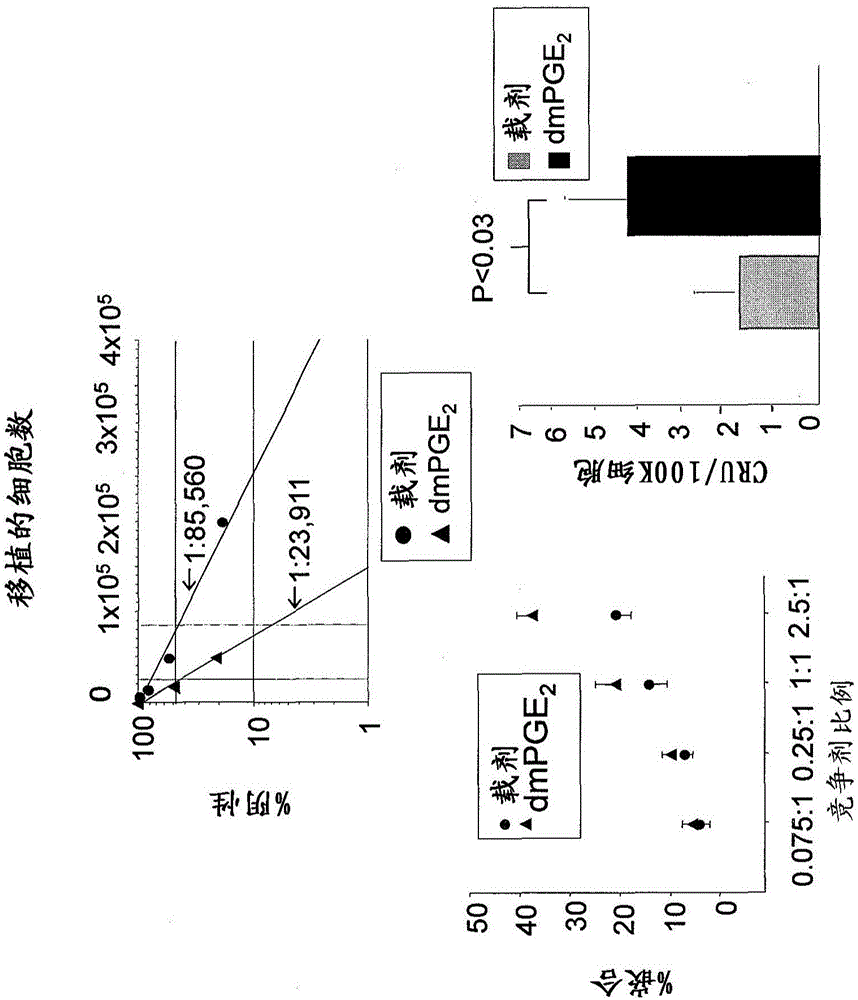
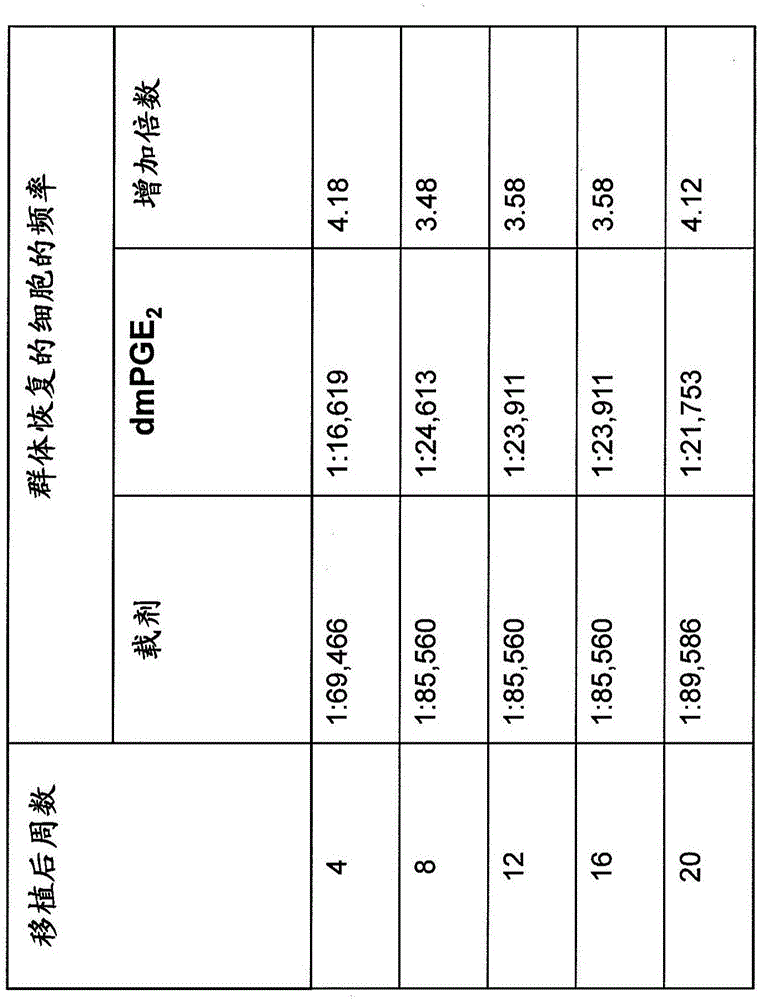
[0098] 1.PGE 2 Increase the frequency and implantation of HSPC in the long-term population
[0099] Using a limited dilution competitive transplantation model, using CD45.2 and CD45.1 congener grafts transplanted into CD45.1 / CD45.2 heterozygous mice to prove that HSPC was exposed to PGE for a short time 2 Produce long-term enhancement of HSPC and Competitive Population Recovery Unit (CRU) frequency. Next reference Figure 1A , With carrier or dmPGE 2 The bone marrow from CD45.1 or CD45.2 mice was treated separately. CD45.1 / CD45.2 hybrid bone marrow cells were used as competitor. The limiting dilution was transplanted into CD45.1 / CD45.2 heterozygous mice irradiated with a lethal dose (1100-cGy, split dose), and the chimerism in PB was analyzed at 20 weeks. A representative flow cytometry chart (bottom panel) for detecting each cell population is shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com