Hot-melt extrusion process for preparing indometacin quick release preparation from multi-element auxiliary materials
A technology of hot melt extrusion and indomethacin is applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, and devices for making medicines into special physical or taking forms, etc. High dispersion of drugs, unfavorable hot-melt extrusion process, difficult drug mixing and other problems, to achieve the effects of short heating time, low cost, and no dust pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
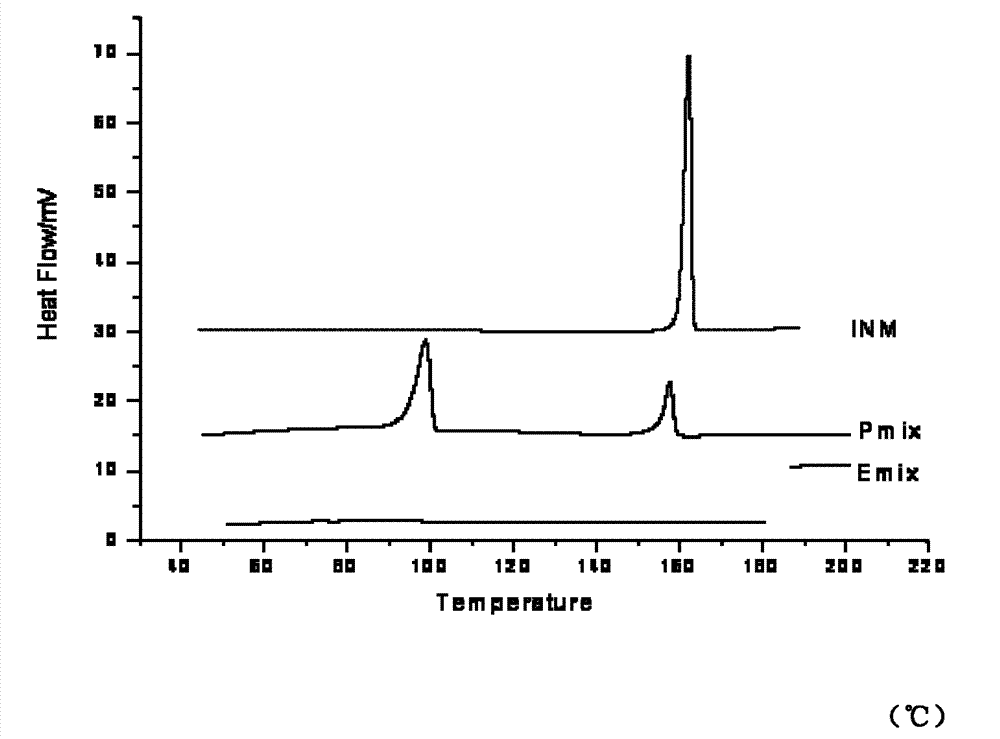
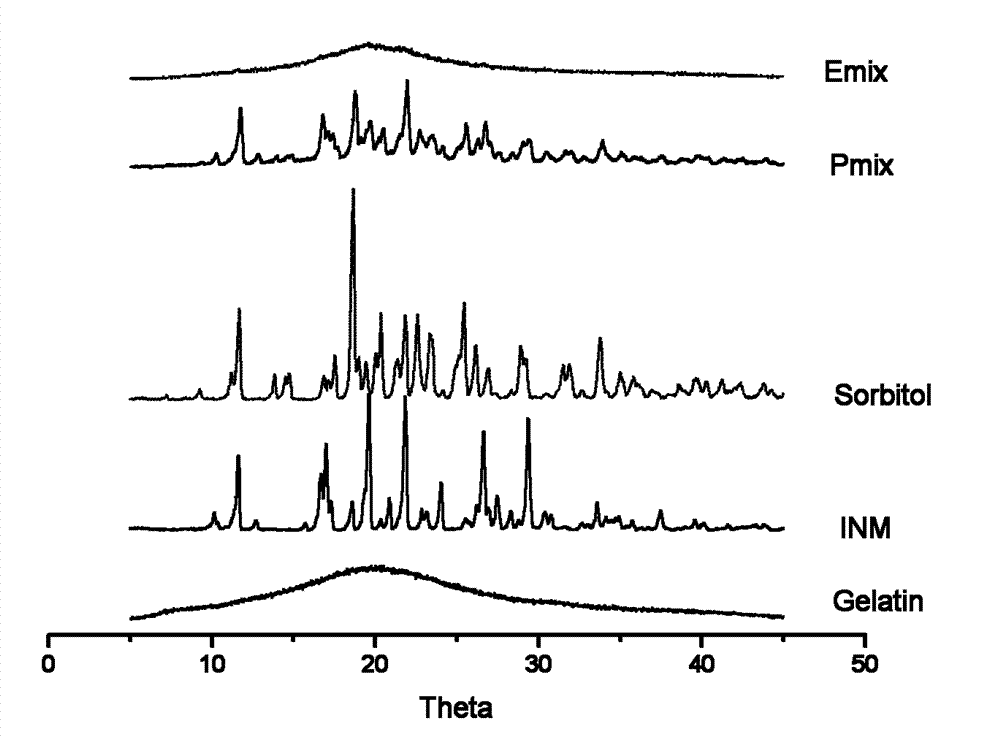
Image
Examples
Embodiment 1
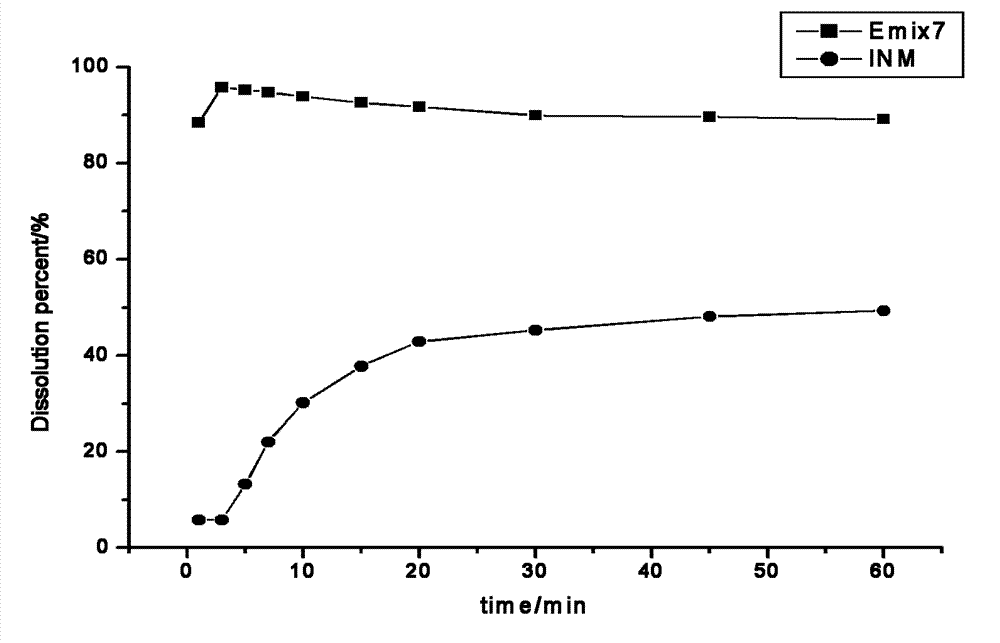
[0029] Indomethacin (25%), gelatin (37.5%), and sorbitol (37.5%) were mixed with a mixer to prepare a physical mixture, and then a twin-screw hot-melt extruder (KEYA TE-20 twin-screw extruder, Nanjing Keya Industrial Co., Ltd., the following 4 examples are the same) Set the temperature at 70°C in the first zone, 140°C in the second zone, and 130°C in the third zone. After the solid is cooled, it is pulverized and passed through a 60-mesh sieve to obtain a solid dispersion of indomethacin. Drug dissolution was 88.5% at 1 minute and 95.8% at 3 minutes. And the bulk drug reaches the highest value only 49% after one hour.
Embodiment 2
[0031] Mix indomethacin (25%), F68 (10%), sorbitol (32.5%), and gelatin (32.5%) with a mixer to prepare a physical mixture, and then set the temperature of the extruder at 110°C in the first zone, The second zone is 110°C, and the third zone is 80°C. After the temperature is stabilized, the physical mixture is added for hot-melt extrusion. The extruded strips are cooled and crushed to pass through a 60-mesh sieve to obtain a solid dispersion of indomethacin. Drug dissolution reached 93% at 1 minute and 95% at 3 minutes.
Embodiment 3
[0033] Mix indomethacin (25%), F68 (5%), IR (12.5%), sorbitol (28.75%), and gelatin (28.75%) with a mixer to prepare a physical mixture, and then set the temperature of the extruder The first zone is 120°C, the second zone is 130°C, and the third zone is 110°C. After the temperature is stabilized, the physical mixture is added for hot-melt extrusion. The extruded strips are cooled and crushed to pass through a 60-mesh sieve to obtain a solid dispersion of indomethacin. Drug dissolution reached 96% at 1 minute and 98% at 3 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com