Quinazolinone derivatives and production method and application thereof
A kind of technology of quinazolinone and derivatives, applied in the field of quinazolinone derivatives and preparation thereof, can solve problems such as no literature reports, and achieve the effects of low production cost, low toxicity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
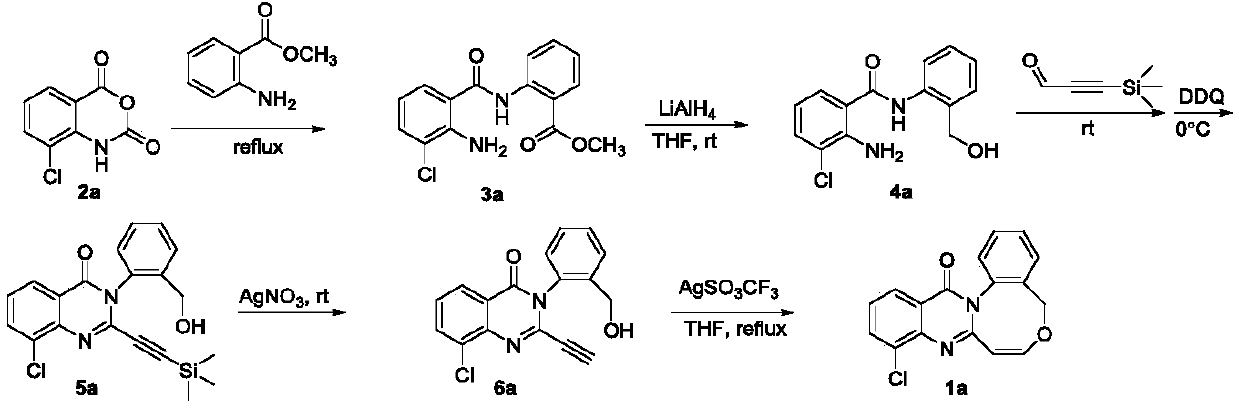
[0025] Synthesis of target product 1a
[0026]
[0027] (1) Under electromagnetic stirring, 4-chloroisatoic anhydride 2a (5mmol, 987mg) and methyl anthranilate (25mmol, 3780mg) were successively added to a 100mL round-bottomed flask, heated to reflux for 12h, and the crude product was Silica gel column chromatography purification (eluent: V 二氯甲烷 :V 石油醚 =1:1) to obtain 1124 mg of white solid compound 3a (yield 74%).
[0028] (2) Under electromagnetic stirring, compound 3a (5 mmol, 1520 mg), tetrahydrofuran (30 mL), lithium aluminum hydride (10 mmol, 380 mg) were sequentially added to a 1000 mL round bottom flask, and the mixture was heated at 0 °C After the reaction was complete, the reaction solution was adjusted to be alkaline with 10% NaOH solution until a white precipitate was formed, then suction filtered, and the filter cake was rinsed three times with dichloromethane, the filtrate was collected, and the solvent was removed under reduced pressure to obtain 840 mg of...
Embodiment 2
[0033] Synthesis of target product 1b
[0034]
[0035](1) Under electromagnetic stirring, 5-fluoroisatoic anhydride 2b (5mmol, 905mg) and methyl anthranilate (25mmol, 3780mg) were successively added to a 100mL round-bottomed flask, heated to reflux for 12h, and the crude product was Silica gel column chromatography purification (eluent: V 二氯甲烷 :V 石油醚 =1:1) to obtain 1036 mg of white solid compound 3b (yield 72%).
[0036] (2) Under electromagnetic stirring, compound 3b (5 mmol, 1440 mg), tetrahydrofuran (30 mL), lithium aluminum hydride (10 mmol, 380 mg) were sequentially added to a 100 mL round bottom flask, and the mixture was heated at 0 °C After the reaction was complete, the reaction solution was adjusted to be alkaline with 10% NaOH solution until a white precipitate was formed, then suction filtered, and the filter cake was rinsed three times with dichloromethane, the filtrate was collected, and the solvent was removed under reduced pressure to obtain 1112 mg of ...
Embodiment 3
[0041] Synthesis of target product 1c
[0042]
[0043] (1) Under electromagnetic stirring, 5-nitroisatoic anhydride (5mmol, 1040mg) and methyl anthranilate (25mmol, 3780mg) were successively added to a 100mL round-bottomed flask, heated to reflux for 12h, and the crude product was Silica gel column chromatography purification (eluent: V 二氯甲烷 :V 石油醚 =1:1), to obtain 2100 mg of white solid compound 3c (yield 67%).
[0044] (2) Under electromagnetic stirring, compound 3c (3.2 mmol, 1000 mg), tetrahydrofuran (30 mL), lithium aluminum hydride (6.4 mmol, 243 mg) were sequentially added to a 250 mL round bottom flask, and the mixture was heated at 0 °C After the reaction was complete, the reaction solution was adjusted to be alkaline with 10% NaOH solution until a white precipitate was formed, then suction filtered, and the filter cake was rinsed three times with dichloromethane, the filtrate was collected, and the solvent was removed under reduced pressure to obtain 470 mg of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com