Pancreatic endocrine progenitor cell therapies for the treatment of obesity and type 2 diabetes (T2D)
A technology of endocrine progenitor cells and type 2 diabetes, applied in the direction of pancreatic cells, endocrine system diseases, animal cells, etc., can solve problems such as treatment difficulties and lack of islets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
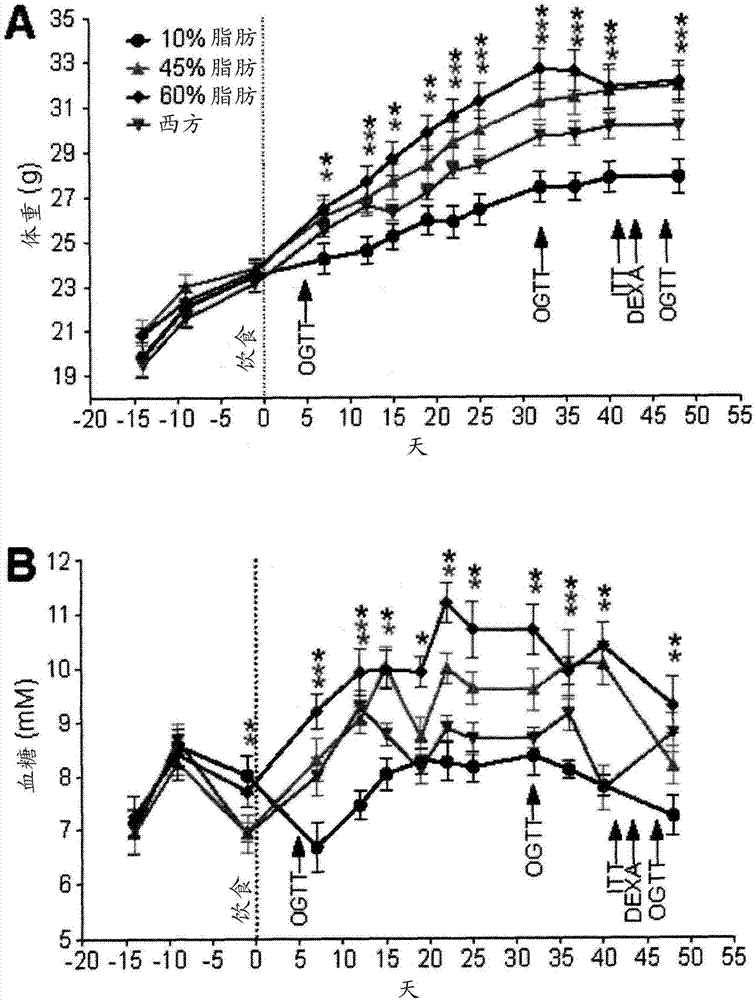
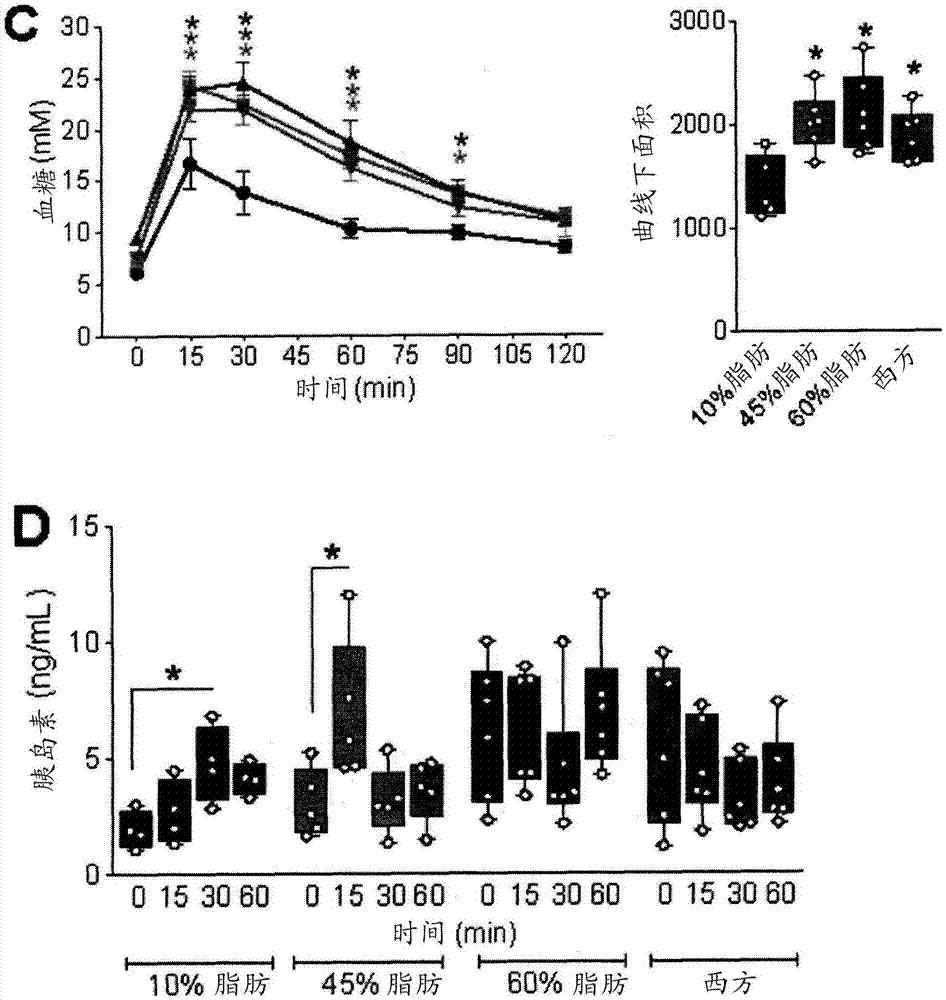
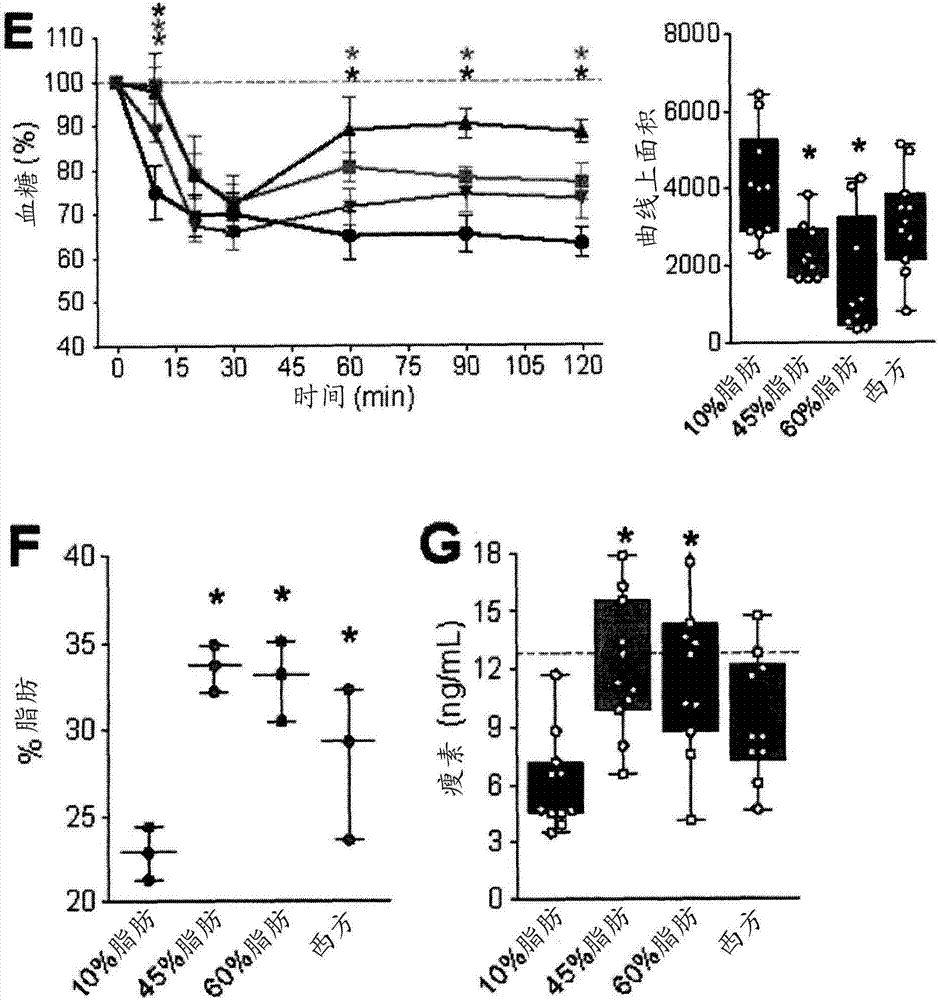
[0197] Example 1: Development of Obesity and Type 2 Diabetes (T2D) Models in Immunodeficient Mice
[0198] For this example, 8 to 10 week old male SCID-beige mice (C.B-Igh-1b / GbmsTac-Prkdc scid -Lyst bg N7; Taconic TM , Hudson, NY) maintained on a 12-h light / dark cycle. All mice received a standard irradiated diet (Harlan Laboratories TM , Teklad Diet TM #2918, Madison, WI) for 2 weeks to allow acclimatization. Mice were placed on 4 different diets (Research Diets TM , NewBrunswick, NJ, one of USA), carried out 36 weeks of research (every diet n=11): 1) "10% fat " control diet (D12450K-10kcal% fat; 70kcal% carbohydrate, no sucrose); 2 ) "45% fat" diet (D12451-45kcal% fat, mainly lard; 35kcal% carbohydrate); 3) "60% fat" diet (D12492-60kcal% fat, mainly lard; 20kcal% carbohydrate) or 4) "Western" diet (D12079B - 41 kcal% fat, mainly milk fat; 43 kcal% carbohydrates, mainly sucrose).
[0199] Using a handheld blood glucose meter (Lifescan TM , Milpitas, California) to m...
Embodiment 2
[0202] Example 2: In Vitro Generation of Pancreatic Endocrine Progenitor Cells from Human Embryonic Stem Cells
[0203] Cells of human embryonic stem cell line H1 (WA01 cells, WiCell Research Institute TM , Madison, WI), as a single cell at 1x10 5 cells / cm 2 Inoculation to mTeSR-1 TM (Stem Cell Technologies TM , Vancouver, BC; Cat.no.05850) in a 1:30 dilution of MATRIGEL TM (Becton Dickinson BioSciences TM , Franklin Lakes, NJ; Catalog ("Cat.") No. 356231) coated dishes. At approximately 70-80% confluency, H1 cell cultures were washed with Mg2+ and Ca2+-free 1X Dulbeccos phosphate-buffered saline (Invitrogen TM , Carlsbad, CA; Cat.No.14190) washing, then at room temperature with 0.02% Versene TM ("EDTA") (Lonza TM , Walkersville, MD; Cat. No. 17-711E) for 12 min. with mTeSR-1 TM Released single cells were washed and spun at 1000 rpm for 5 min. The resulting cell pellet was resuspended in the ROCK inhibitor Y-27632 supplemented with 10 μM TM (Sigma-Aldrich TM , St....
Embodiment 3
[0211] Example 3: Exposure to HFD does not affect in vivo function of hESC-derived endocrine cells
[0212] According to the following steps, the pancreatic endocrine precursor cells of Example 2 were encapsulated in 20 μl Theracyte TM Macroencapsulation device (TheraCyte Inc. TM , Laguna Hills, CA). Will be about 5x10 6 Endocrine precursor cells (in the form of cell clusters) are placed in a positive displacement pipette. Using gentle pressure, place the capillary tip / piston tip containing the cells snugly in the center of the 24 gauge catheter and dispense the cells from the positive displacement pipette through the catheter into the device. Titanium barbs are used to seal the unit. The encapsulated pancreatic endocrine precursor cells were then transplanted subcutaneously into 7 SCID-beige mice from each of the 4 dietary regimens. All mice were anesthetized with inhalable isoflurane, and transplant recipients received approximately 5x10 6 pancreatic endocrine precurso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com