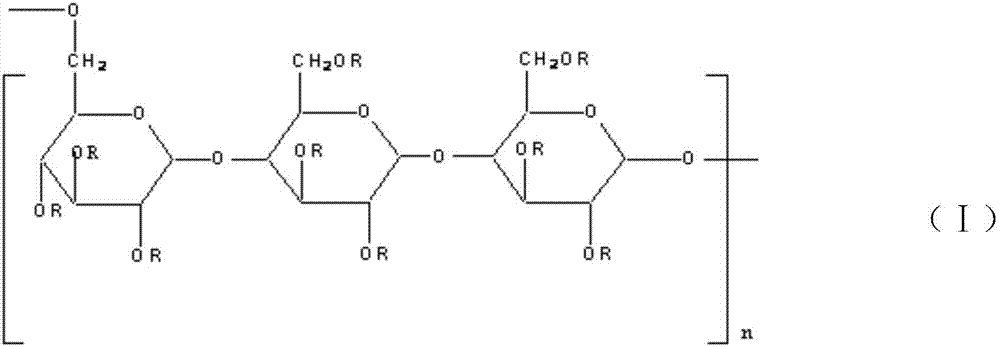
Acid-resistant Pulullan derivative, and preparation method thereof
A pullulan polysaccharide and derivative technology, applied in the chemical industry, can solve problems such as easy dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
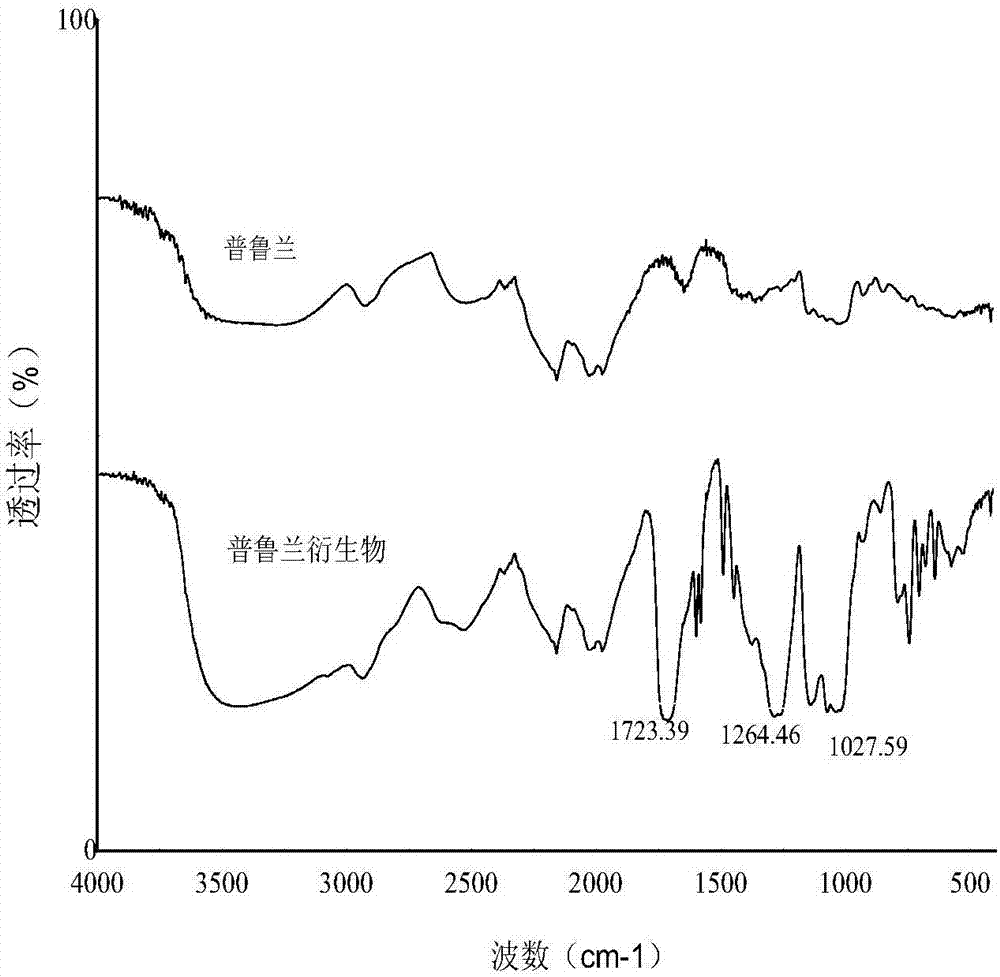
[0029] 15g pullulan, 30g phthalic anhydride, 55g pyridine, 105ml N,N-dimethylformamide. When the temperature rises to 80°C, the timed reaction is started for 12h. After the completion of the reaction, the reaction solvent was evaporated in vacuum, and the product was poured into ice water for washing. Wash repeatedly until the wash solution is neutral. Filtration to obtain wet product, freeze-dried to obtain product. The structural infrared characterization of the product is as follows figure 1 As shown, the mass percentage of benzoic acid formyl group is 35%, and the pullulan derivative remains intact at low pH (pH=1.5-4), but dissolves at higher pH (pH=5-7).
Embodiment 2
[0031] 15g pullulan, 10g phthalic anhydride, 25g pyridine, 35ml N,N-dimethylformamide. When the temperature rises to 50°C, the timed reaction starts for 48h. After the completion of the reaction, the reaction solvent was evaporated in vacuum, and the product was poured into ice water for washing. Wash repeatedly until the wash solution is neutral. Filtration to obtain the wet product, drying at 40°C to obtain the product. The mass percentage of benzoic acid formyl group was 21%, and the pullulan derivative remained intact at low pH (pH=1.5-4.5), but dissolved at higher pH (pH=5-7).
Embodiment 3
[0033] 15g pullulan, 70g phthalic anhydride, 200g triethylamine, 250ml N,N-dimethylformamide. When the temperature rises to 60°C, the timed reaction is started for 24h. After the completion of the reaction, the reaction solvent was evaporated in vacuum, and the product was poured into ice water for washing. Wash repeatedly until the wash solution is neutral. Filtration to obtain wet product, freeze-dried to obtain product. The mass percentage of benzoic acid formyl group was 65%, and the pullulan derivative remained intact at low pH (pH=1.5-5), but dissolved at higher pH (pH=5-7).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com