Preparation method and application of soluble euglena polysaccharide
A technology of soluble and euglena, applied in the field of biomedicine, can solve the problem of low solubility of euglena, achieve significant anti-inflammatory effect, excellent suspension, and relieve focal spongy edema
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] A preparation method of soluble Euglena polysaccharide, the method is method 1 or method 2, wherein, the method 1 comprises the following steps:
[0043] (1) Alkali dissolving: use sodium hydroxide solution or potassium hydroxide solution or calcium hydroxide solution or barium hydroxide lye solution of pH>12 to dissolve euglena, and mechanically stir until the solid of euglena disappears;
[0044] (2) Acid cross-linking: add acid solution dropwise, as the pH decreases, gelatinous precipitation appears in the solution, add acid solution dropwise until the pH of the solution is less than or equal to 7;
[0045] (3) Oxidative bond breaking: add oxidant hydrogen peroxide or sodium peroxide or calcium peroxide solution or hypochlorous acid or sodium hypochlorite until the peroxide concentration in the solution is 0.01g / L~0.1g / L or the available chlorine content is 0.1g / L~1g / L, stir until the gelatinous precipitate formed in step (2) in method 1 disappears, so that the glyco...
Embodiment 1
[0063] Take 10 g of Euglena, dissolve with 100 mL of NaOH (pH=12.5) with a concentration of 1 mol / L, stir with a magnetic stirrer to make the Euglena powder disappear and dissolve completely. Then, 5% hydrochloric acid was added dropwise while stirring continued. After the pH was lowered, lumpy and flocculent gels were precipitated, and acid was continued to be added until the pH was 6. Then add 15ml of 30% (mass fraction) hydrogen peroxide to break the bond. At this time, the solution produces a large number of bubbles, supplemented by magnetic stirrer stirring, the gel block disappears in about a few minutes, and the solution becomes clear. The volume of the solution was 120 ml, and 1 mol / L NaOH solution was added dropwise. After the pH was raised to 7, 2 mg of ascorbic acid was added to stop the oxidation of hydrogen peroxide.
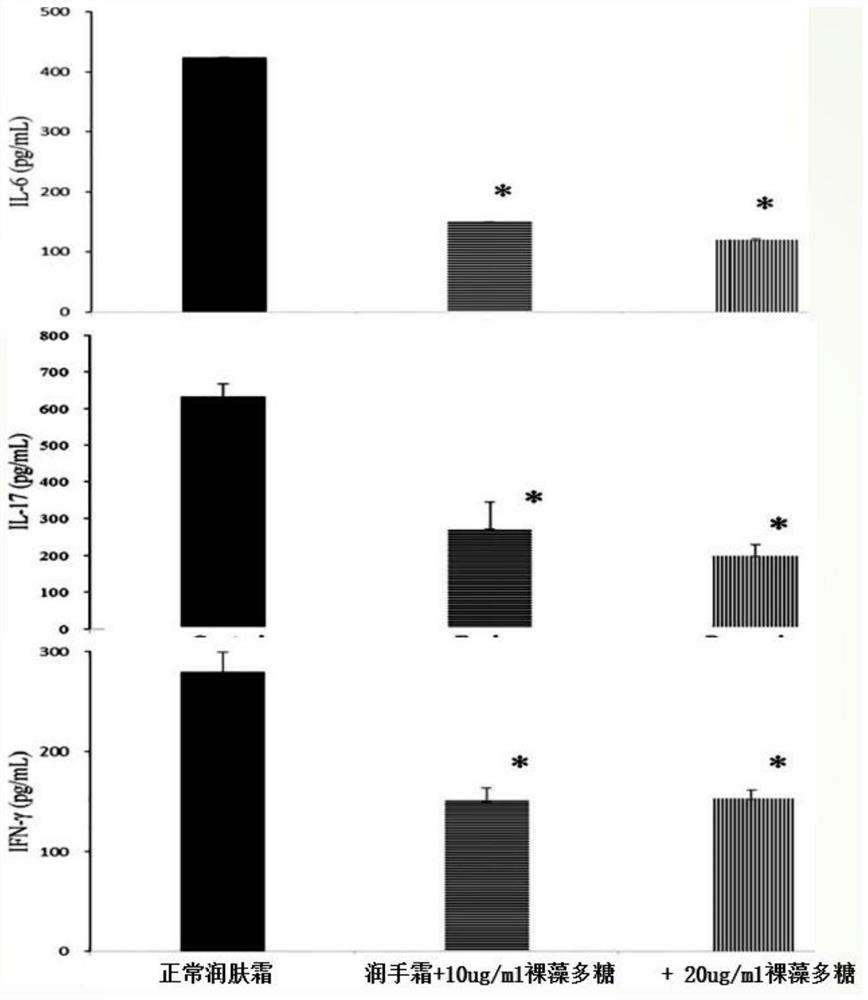
[0064] After standing for 24, the solution did not appear to be figure 2 The phenomenon that the non-soluble Euglena polysaccharide precipitates ...
Embodiment 2
[0072] Take 10g of Euglena polysaccharide, dissolve it with 100mL NaOH (pH=12.5) with a concentration of 1mol / L, stir with a magnetic stirrer, the Euglena polysaccharide powder completely disappears and dissolves completely within one minute. Then, 5% hydrochloric acid was added dropwise while stirring was continued. When the pH was lower than 13, gel precipitation occurred, and acid was continued to be added until the pH was 6. Add 100ml of 30% (mass fraction) sodium hypochlorite to make it oxidize and break bonds. At this time, the solution produces a lot of bubbles. Stir with a magnetic stirrer until the gel completely disappears and the solution becomes clear. Add 5% (mass fraction) hydrochloric acid dropwise. , after adjusting the pH to 6, 2 mg of ascorbic acid was added to stop the oxidation of sodium hypochlorite.
[0073] The SBA-50B glucose analyzer was used to measure the monosaccharide concentration in the solution. The measurement results showed that the glucose mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com