Preparation method of gamma-Fe2O3 nano material
A nanomaterial, 9H2O technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve problems such as operator and environmental hazards, toxicity, and large amount of dimethylformamide used , to achieve the effect of high product purity, simple preparation method and few operation links
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
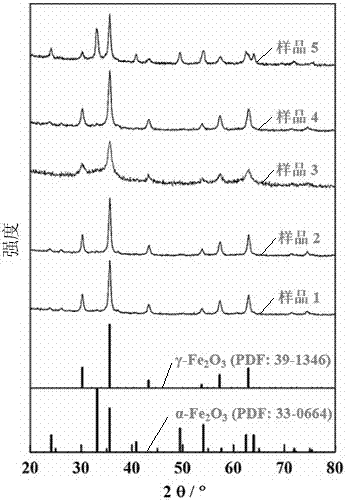
[0018] Embodiment 1: weigh 40 grams of Fe (NO 3 ) 3 •9H 2 0. Put 18 grams of L-tartaric acid into a glass beaker, then weigh 12 grams of water and pour it into the above-mentioned beaker, stir and dissolve with a glass rod to obtain a reddish-brown viscous liquid, put it into an oven at 88°C and dry it for 16 hours; then The dried solid was put into a muffle furnace and roasted at 350°C for 3 hours to obtain red powder sample 1, namely γ-Fe 2 o 3 Nanoparticles. Using the PNAlytical X'Pert X-ray diffractometer to analyze the crystal structure of sample 1, compared with the standard spectrum, only γ-Fe was detected 2 o 3 , the results are shown in the attached figure 1 , figure 1 To five samples prepared by embodiment 1 to embodiment 5 respectively, utilize PNAlytical X'Pert X-ray diffractometer to analyze their diffraction peaks, and with standard substance α-Fe 2 o 3 (PDF:33-0664) and γ-Fe 2 o 3 (PDF: 39-1346) and their crystal structures were compared, and the gra...
Embodiment 2
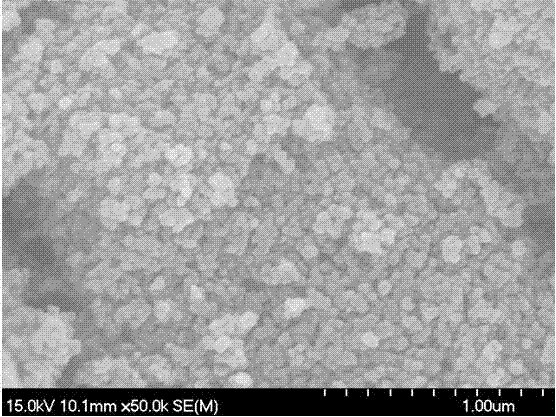
[0019] Embodiment 2: weigh 40 grams of Fe (NO 3 ) 3 •9H 2 O. Put 18 grams of L-tartaric acid into a glass beaker, then weigh 15 grams of water and pour it into the above-mentioned beaker, stir and dissolve with a glass rod until it becomes a reddish-brown viscous liquid, put it into a 90°C oven and dry it for 12 hours; then The dried solid was put into a muffle furnace and calcined at 380°C for 5 hours to obtain red powder sample 2. Using the PNAlytical X'Pert X-ray diffractometer to analyze the crystal structure of sample 2, compared with the standard spectrum, only γ-Fe was detected 2 o 3 , the results are shown in the attached figure 1 , the grain size calculated by using the strongest diffraction peak at 35.63° and the Scherer equation is 24 nanometers; the morphology of sample 2 is observed with a Hitachi S-4700II scanning electron microscope, and its grain size is about 50 nanometers. The results are shown in the attached figure 2 .
Embodiment 3
[0020] Embodiment 3: weigh 40 grams of Fe (NO 3 ) 3 •9H 2O. Put 18 grams of L-tartaric acid into a glass beaker, then weigh 15 grams of water and pour it into the above-mentioned beaker, stir and dissolve with a glass rod until it becomes a reddish-brown viscous liquid, put it into a 92°C oven and dry it for 12 hours; then The dried solid was put into a muffle furnace and calcined at 280° C. for 6 hours to obtain a red powder sample 3 . Using the PNAlytical X'Pert X-ray diffractometer to analyze the crystal structure of sample 3, compared with the standard spectrum, only γ-Fe was detected 2 o 3 , the results are shown in the attached figure 1 , using the strongest diffraction peak at 35.57° and the Scherer equation to calculate the grain size to be 18 nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com