Alizarin liposome and preparation method thereof
A technology of alizarin lipid and alizarin, which is applied in the field of alizarin liposome and its preparation, can solve the problems of short half-life in vivo, low bioavailability, cardiotoxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
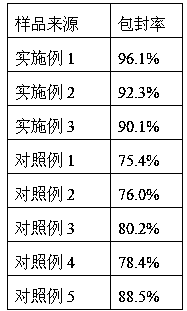
Embodiment 1
[0020] A preparation method of rubidin liposome, the steps are as follows:
[0021] (1) In terms of mass ratio, weigh 10 mg of madderin and 350 mg of PEG-modified lipid derivative, the PEG-modified lipid derivative is PEG2000-distearoylphosphatidylethanolamine;
[0022] (2) Preparation of blank liposome suspension by injection method: Dissolve PEG-modified lipid derivatives in absolute ethanol to make a lipid solution, absorb the lipid solution and inject slowly into 5mL pH=4, concentration 300mmol / In the citrate-citrate sodium buffer solution (hydration medium) of L, continue stirring to remove ethanol after injecting, and obtain blank liposome suspension after ultrasonic dispersion;
[0023] (3) Ultrasonically disperse rubikin in 5mL of absolute ethanol to prepare rubikin ethanol dispersion;
[0024] (4), mixing the blank liposome suspension obtained in step (2) and the ethanol dispersion of rubidin obtained in step (3), supercritical CO at 50°C and 8Mpa 2 After 5 minutes...
Embodiment 2
[0026] A preparation method of rubidin liposome, the steps are as follows:
[0027] (1) In terms of mass ratio, weigh 10 mg of madderin and 300 mg of PEG-modified lipid derivative, the PEG-modified lipid derivative is PEG2000-distearoylphosphatidylethanolamine;
[0028] (2) Preparation of blank liposome suspension by reverse evaporation method: dissolve PEG-modified lipid derivatives in absolute ethanol to make a lipid solution, then add 5 mL of citrate with pH=2.7 and a concentration of 200 mmol / L In the acid-citrate sodium buffer solution (hydration medium), high-speed shearing makes it form stable W / O colostrum, and then evaporates the organic solvent under reduced pressure to obtain a blank liposome suspension;
[0029] (3) Ultrasonically disperse rubikin in 3.33mL absolute ethanol to prepare rubikin ethanol dispersion;
[0030] (4), mixing the blank liposome suspension obtained in step (2) and the ethanol dispersion of rubidin obtained in step (3), supercritical CO at 55...
Embodiment 3
[0032] A preparation method of rubidin liposome, the steps are as follows:
[0033] (1) In terms of mass ratio, weigh 10 mg of madderin and 400 mg of PEG-modified lipid derivative, the PEG-modified lipid derivative is PEG5000-distearoylphosphatidylglycerol;
[0034] (2) Preparation of blank liposome suspension by spray drying method: dissolve PEG-modified lipid derivatives in absolute ethanol to make a lipid solution, spray dry the lipid solution to obtain a lipid powder, and use 6 mL pH = 5.2, citrate-citrate sodium buffer solution (hydration medium) with a concentration of 400mmol / L hydrates the lipid powder to prepare a blank liposome suspension;
[0035] (3) Ultrasonically disperse rubikin in 4mL of absolute ethanol to prepare rubikin ethanol dispersion;
[0036] (4), mixing the blank liposome suspension obtained in step (2) and the ethanol dispersion of rubidin obtained in step (3), supercritical CO at 50°C and 10Mpa 2 After 3 minutes of treatment, the rubiacin liposome...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com