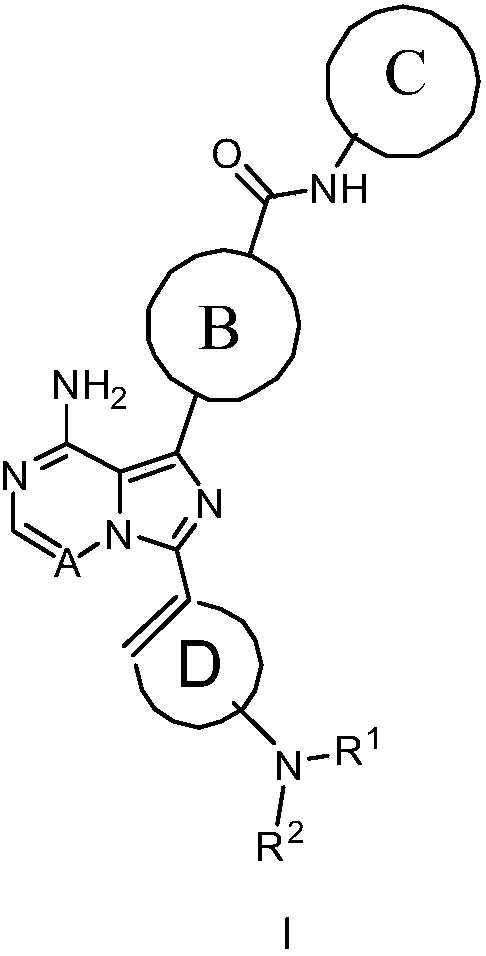
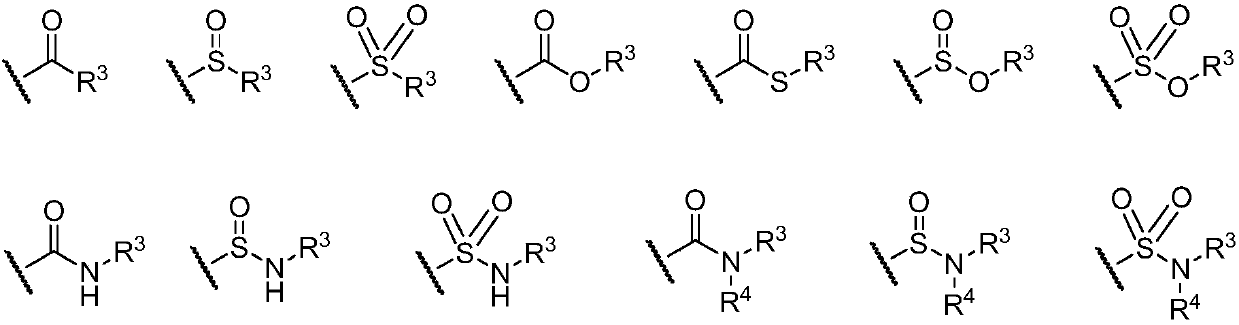
Bruton tyrosine kinase inhibitor
A solvate and compound technology, applied in the field of medicine, can solve the problems of no obvious prolongation of the overall survival of patients, high toxicity, and frequent occurrence of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0187] The synthetic route of compound I-1
[0188]
[0189] 4-(8-Amino-1-(4-(pyridin-2-ylcarbamoyl)phenyl)imidazo[1,5-a]pyrazin-3-yl)cyclohex-3-enylcarbamate Benzyl ester 24-1
[0190]
[0191] Intermediate A-1 (30mg, 0.06mmol), 4-(pyridin-2-ylcarbamoyl)phenylboronic acid B-1 (18mg, 0.074mmol), Pd(PPh 3 ) 4 (7mg, 0.006mmol) and Cs 2 CO 3 (25mg, 0.074mmol) in DME: H 2 The mixture in a mixed solvent of O (2.5ml:0.5ml) was heated to 80°C overnight. It was concentrated and purified by column chromatography, eluting with EA-MeOH / DCM (1:40) to give compound 24-1 (25 mg, 75%).
[0192] LC-MS m / z=560.2[M+1] + .
[0193] 4-(8-Amino-3-(4-aminocyclohex-1-enyl)imidazo[1,5-a]pyrazin-1-yl)-N-(pyridin-2-yl)benzamide 25-1
[0194]
[0195] To a solution of compound 24-1 (20 mg, 0.036 mmol) in DCM (5 ml) was added HBr (33% in AcOH) solution (2 drops). The suspension was stirred at room temperature for 2 hours. The solvent was evaporated to give the crude product 25-1 hydrob...
Embodiment 2
[0200] 4-(8-Amino-3-(4-but-2-ynamidocyclohex-1-enyl)imidazo[1,5-a]pyrazin-1-yl)-N-(4-fluoro Pyridin-2-yl)benzamide I-2
[0201]
[0202] Synthesis of Compound I-2 Using intermediates A-1 and B-2 as raw materials, it was synthesized using Synthesis Method 2 of I-1. Compound I-2 was obtained after purification. LC-MS m / z=510.2[M+1] + .
Embodiment 3
[0204] 4-(8-amino-3-(4-but-2-yne amidocyclohex-1-enyl)imidazo[1,5-a]pyrazin-1-yl)-N-(4-( Trifluoromethyl)pyridin-2-yl)benzamide I-3
[0205]
[0206] Synthesis of Compound I-3 Using intermediates A-1 and B-3 as raw materials, it was synthesized using Synthesis Method 2 of I-1. Compound 1-3 was obtained after purification. LC-MS m / z=560.2[M+1] + .
[0207] The synthesis of compound I-3 can be synthesized by synthetic method 3
[0208]
[0209] 4-(8-(2,4-dimethoxybenzylamino)-3-(4-(tert-butoxycarbonylamino)cyclohex-1-enyl)imidazo[1,5-a]pyridine Oxin-1-yl) methyl benzoate 5-3
[0210]
[0211] Methyl 4-(8-(2,4-dimethoxybenzylamino)-3-bromoimidazo[1,5-a]pyrazin-1-yl)benzoate M (721 mg, 1.45 mmol), tert-butyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxa)boran-2-yl)cyclohex-3-enylcarbamate L-3 (562mg, 1.74mmol), Pd[PPh 3 ] 4 (168mg, 0.145mmol) and Cs 2 CO 3 (706 mg, 2.17 mmol) was stirred in dioxane (30 mL) at 90°C for 2.5 hours. Concentrated and dissolved in DCM (100 mL)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com