Method for preparing photoactive 2 nitrophenol through catalyzing by utilizing biological enzyme
A technology for catalytic preparation and nitrophenol, applied in biochemical equipment and methods, enzymes, fermentation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of pod peroxidase.
[0046] After 10kg of fresh soybean pods are crushed with a pulverizer, the particle size is less than 1mm, add 50L deionized water and soak for 4 hours at room temperature. After the soaking liquid is filtered, the initial extract is obtained, the volume of the initial extract is V; solid sulfuric acid is added to the initial extract during stirring Ammonium, make 45% saturation, and the volume is V1; add 0.3V of pre-cooled acetone while continuing to stir, and centrifuge at 5000 rpm for 10 minutes to obtain supernatant 1, and add solid ammonium sulfate to supernatant 1, According to the volume of V1, add to 75% saturation, centrifuge at 5000 rpm for 10 minutes; at this time, a layer of precipitate will form at the interface between the water phase and the acetone phase, namely precipitation 1, take precipitation 1, add precipitation 1 volume 5 times Dissolve the precipitate 1 in deionized water. After desalting by ultrafiltration, centrifu...
Embodiment 2
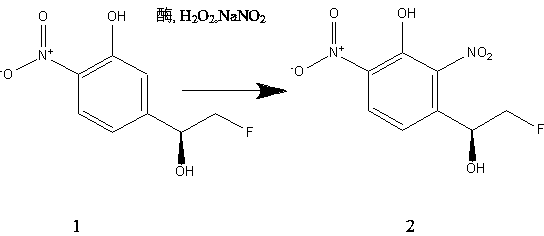
[0050] The preparation of (S)-3-(2-fluoro-1-hydroxyethyl)-2,6-2 nitrophenol was catalyzed by pod peroxidase.
[0051] In a 1L reactor, the pod peroxidase of Example 1 was used for the biocatalytic reaction, the substrate was (S)-5-(2-fluoro-1-hydroxyethyl)-2-nitrophenol, the target product It is (S)-3-(2-fluoro-1-hydroxyethyl)-2,6-2 nitrophenol, the ee% of the substrate is 95-99.5%; the reaction medium is a water-organic solvent two-phase system, The aqueous phase is a phosphate buffer with pH 7.0, the organic solvent is isobutanol, the aqueous phase / organic phase=8 / 2, the volume of the reaction solution is 700-750ml, the substrate is dissolved in isobutanol, and then added to the reaction The reaction temperature is 49-51℃, the reaction time is 8-9h, the rotating speed of the stirring blade is 400-450rpm, the concentration of pod peroxidase is 4-5mU / ml, the concentration of substrate is 30mM, NaNO 2 The concentration is 350mM, H 2 O 2 Adopt the feeding method of feeding, add H a...
Embodiment 3
[0054] Use commercially available horseradish peroxidase, lactoperoxidase, chloroperoxidase (from the marine fungus Caldariomyces fumago), and soybean hull peroxidase as catalysts to prepare (S)-3-(2-fluoro -1-Hydroxyethyl)-2,6-2 Nitrophenol.
[0055] The conditions of the catalytic reaction are the same as in Example 2, but the reaction temperature and pH are adjusted according to different enzymes. The experimental results are that the substrate conversion rate is 17-62%, the target product ee% is 95-99.5%, and the target product yield is 14 -49%. It can be seen that only the pod peroxidase has the best catalytic effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com