Application of miR-214 antagonist in preparing drug for relieving kidney tubule lesion-related diseases caused by proteinuria
A related disease, 1. mir-214 technology, applied in drug combinations, urinary system diseases, pharmaceutical formulations, etc., can solve problems such as no miR-214 antagonists yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
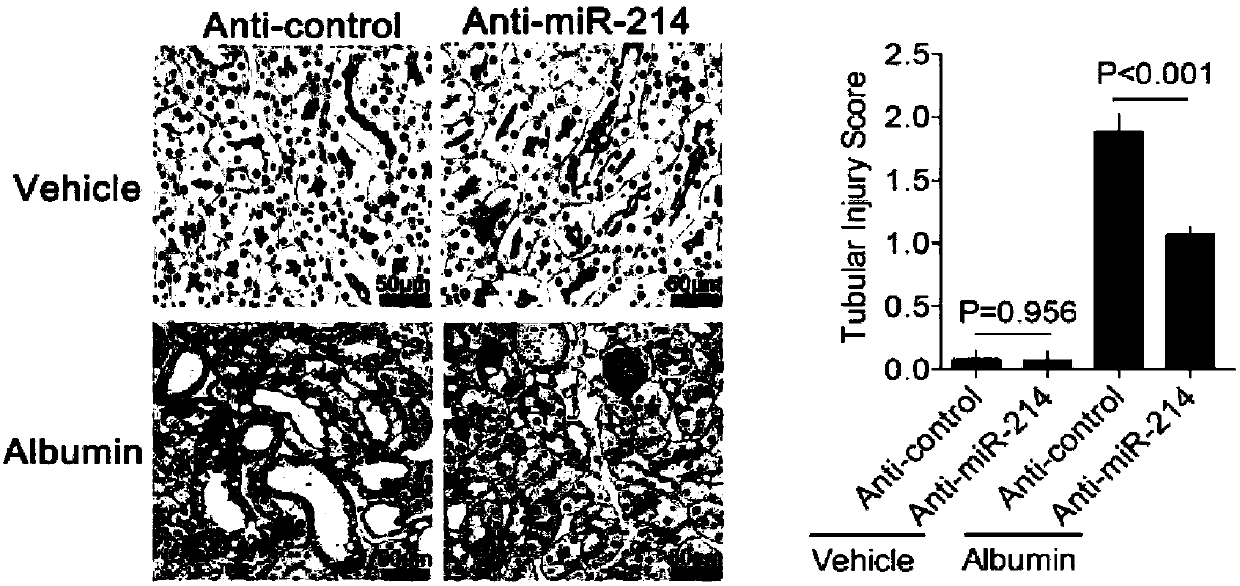
[0044] Example 1 Effect of miR-214 antagonists on albumin-induced renal function.
[0045] Male C57BL / 6 mice weighing 18-22 g were divided into 4 groups, namely, the blank control group (vehicle+anti-control (anti-control is an oligonucleotide sequence without any function: 5'-3'ACGTCTATACGCCCA ), miR-214 antagonist group (vehicle+anti-miR-214), albumin model group (Albumin+anti-control) and miR-214 antagonist+albumin group (anti-miR-214+ albumin).
[0046] Albumin model group: intraperitoneal injection, gradually increasing body weight from 2 mg / g to 10 mg / g body weight on the fifth day, and maintaining intraperitoneal injection of 10 mg / g body weight for the next 6 days, a total of 11 days, the mice were killed on the 12th day, and the kidneys were collected organize.
[0047] miR-214 antagonist treatment group (i.e. miR-214 antagonist + albumin group): the miR-214 antagonist was formulated with RNAase-removed physiological saline to a concentration of 1 μg / μL, and administ...
Embodiment 2
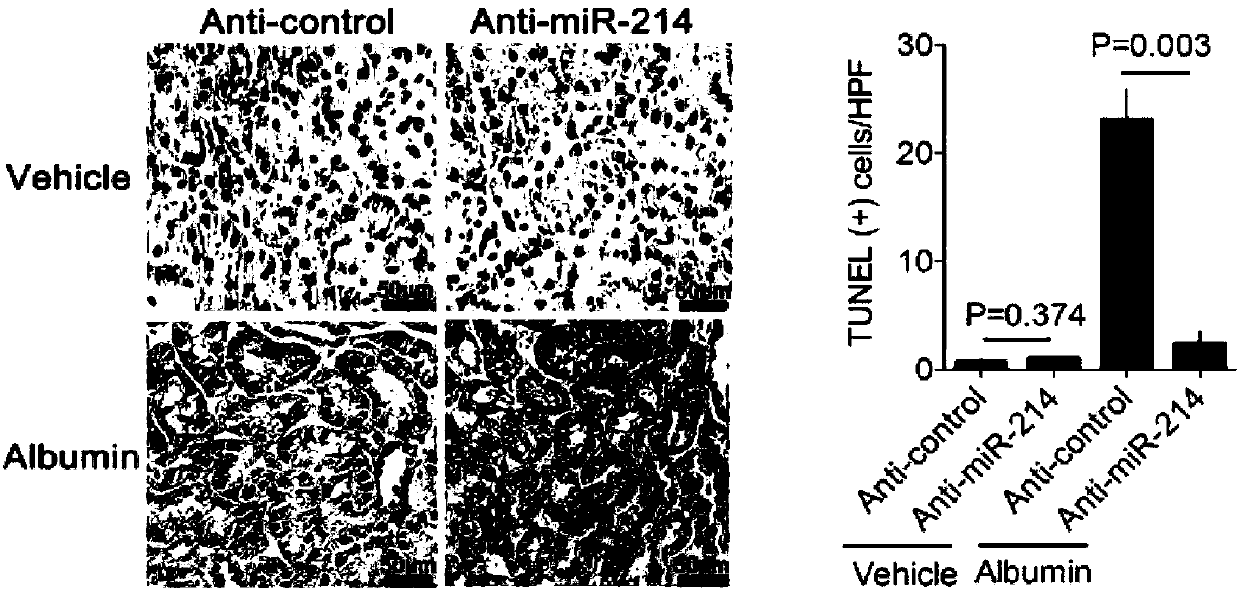
[0051] Example 2 Effects of miR-214 antagonists on albumin-induced tubular cell apoptosis.
[0052] TUNEL (TdT-mediated dUTPNick-End Labeling) positive tubular cells in albumin-induced renal injury were detected by tissue immunohistochemistry.
[0053] Such as figure 2 As shown, in the albumin-induced kidney injury model, the blank control group (vehicle+anti-control), the miR-214 antagonist group (vehicle+anti-miR-214) had no obvious TUNEL positive cells under the microscope, and the albumin model group (Albumin+anti-control) had 21.4 ± 3.2 positive cells in each high-power field, and miR-214 antagonist treatment group (anti-miR-214+albumin) had 3.5±0.82 positive cells in each high-power field (n =6).
[0054] The results showed that administration of miR-214 antagonist alone had no effect on tubular cells. Albumin can induce a large amount of apoptosis of tubular cells, compared with the blank control group, p<0.05. And miR-214 antagonist can significantly reduce apopto...
Embodiment 3
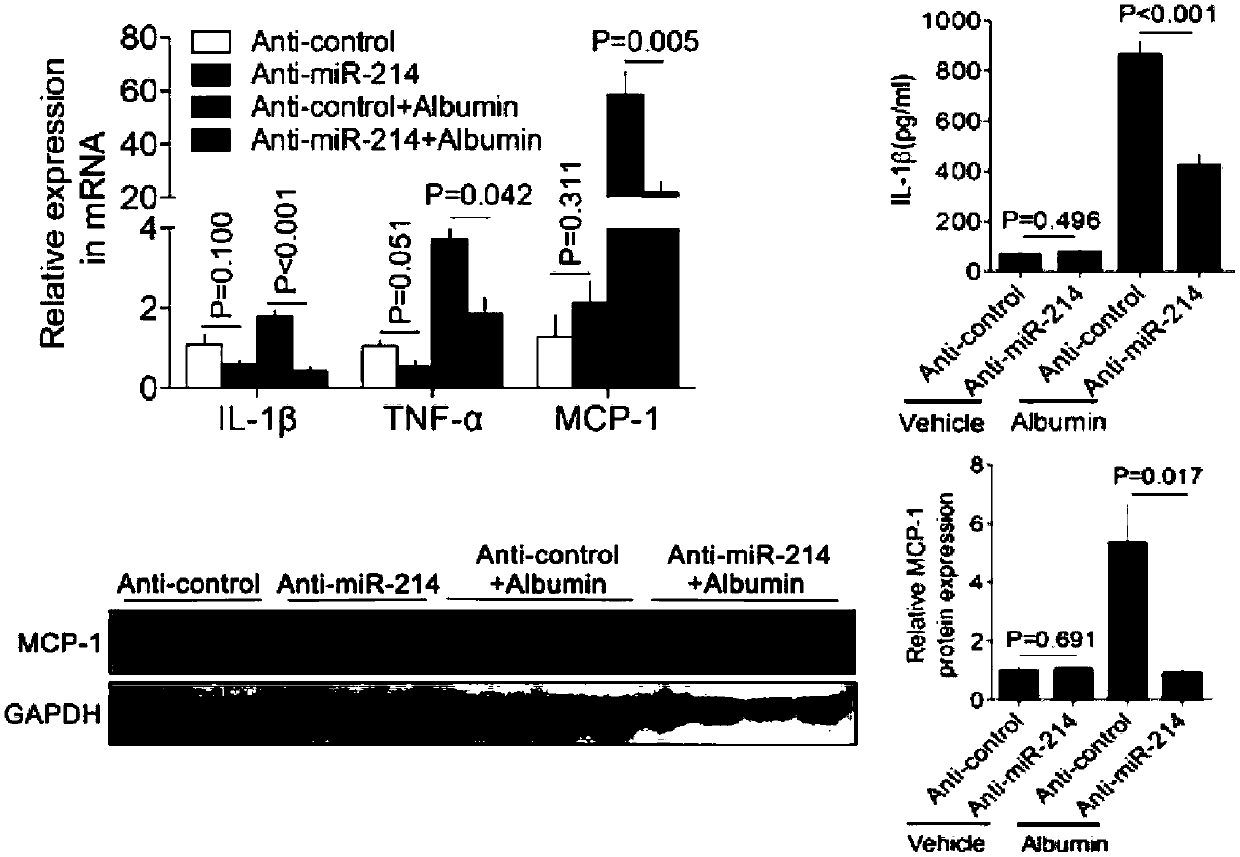
[0055] Example 3 Effects of miR-214 antagonists on albumin-induced renal inflammation.
[0056] The expressions of renal inflammatory factors IL-1β, TNF-α and MCP-1 were detected by PCR, and the secretion of IL-1β in serum was detected by Western detection of MCP-1 and ELISA.
[0057] Compared with the blank control group, administration of miR-214 antagonist alone did not increase the expression of inflammatory factors, and the expression of inflammatory factors in the albumin model group increased significantly, p<0.05. And miR-214 antagonist can significantly reduce the expression of inflammatory factors, compared with albumin group, p<0.05.
[0058] The results showed that albumin could induce the increased expression of renal inflammatory factors, while miR-214 antagonist could inhibit the expression of inflammatory factors.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com