Fluorescent cell counting detection kit for detecting tumor markers
A technology for detection kits and tumor markers, which is applied in the field of biomedical detection and can solve problems such as lack of system, mature products, and no patent authorization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
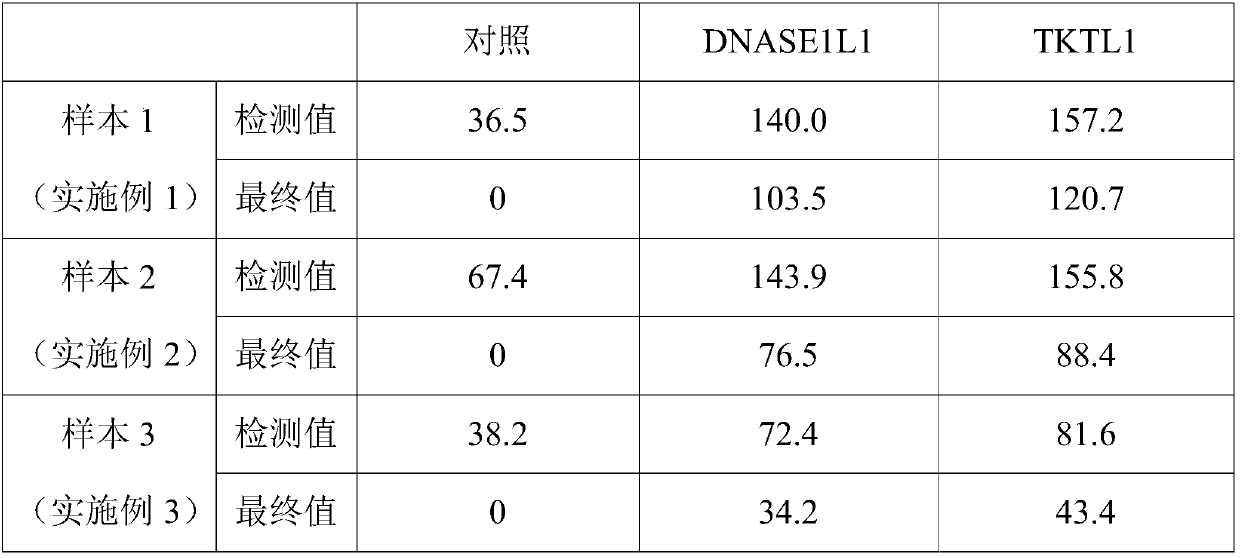
Embodiment 1
[0045] 1. Take 200ul of the peripheral blood sample of the subject to be tested, add 40ul of anti-CD14-FITC (BD company, product number 555397) and 10ul of anti-CD16-APC (BD company, product number 561304), and incubate at room temperature for 15 minutes in the dark;
[0046] 2. Add 200 ul of fixative and incubate at room temperature in the dark for 8 minutes; the components of the fixative are: formaldehyde with a volume percentage of 0.8%, and methanol with a volume percentage of 0.30%;
[0047] 3. Add 3 mL of lysate to the sample, vortex to mix, and incubate in the dark for 15 minutes to lyse red blood cells; the lyse contains 8mM Tris-HCL, 8mM NaH 2 PO 4 and / or NaHPO 4 , 140mM NaCl, 1.3% by volume TritonX-100, 8mM Na 4 P 2 o 7 10H 2 O, pH=7.2;
[0048] 4. Centrifuge at 700g for 6 minutes to remove the supernatant; pour off the supernatant slowly, absorb the residual liquid with paper, and resuspend the cells by vortexing;
[0049] 5. Add 80ul of blocking and punchin...
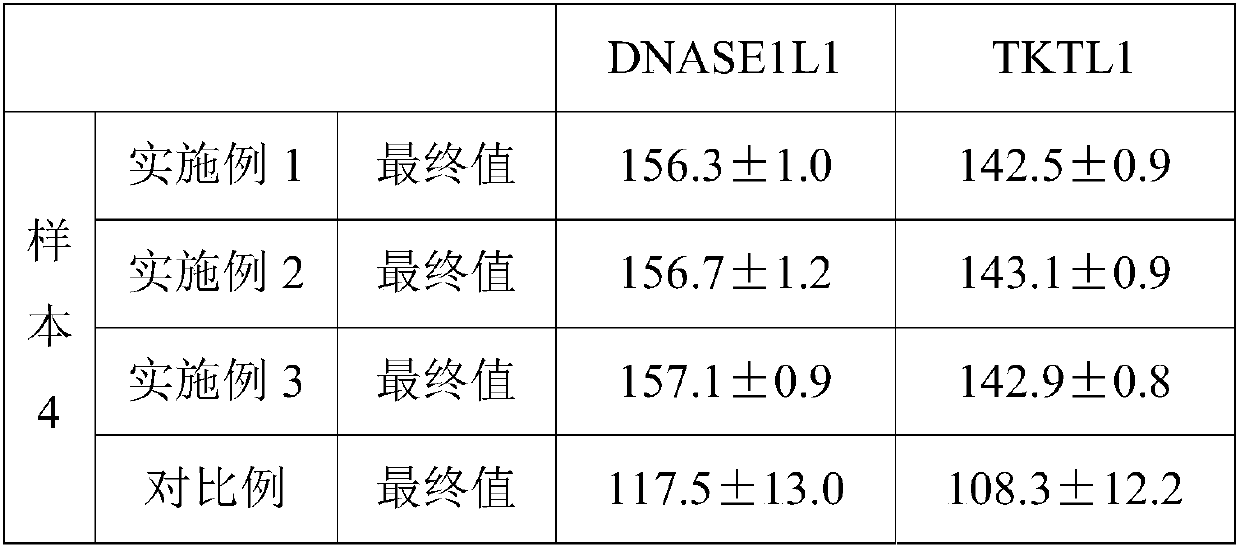
Embodiment 2
[0058] 1. Take 200ul of the peripheral blood sample of the subject to be tested, add 40ul of anti-CD14-FITC (BD company, product number 555397) and 10ul of anti-CD16-APC (BD company, product number 561304), and incubate at room temperature for 15 minutes in the dark;
[0059] 2. Add 200 ul of fixative and incubate at room temperature in the dark for 6 minutes; the components of the fixative are: formaldehyde with a volume percentage of 1.2%, and methanol with a volume percentage of 0.38%;
[0060] 3. Add 3 mL of lysate to the sample, vortex to mix, and incubate in the dark for 15 minutes to lyse the red blood cells; the lyse contains 12mM Tris-HCL, 12mM NaH 2 PO 4 and / or NaHPO 4 , 120mM NaCl, 0.7% by volume Triton X-100, 12mM NaCl 4 P 2 o 7 10H 2 O, pH=7.7;
[0061] 4. Centrifuge at 900g for 4 minutes to remove the supernatant; slowly pour off the supernatant, absorb the residual liquid with paper, and vortex to resuspend the cells;
[0062] 5. Add 120ul of blocking and...
Embodiment 3
[0071] 1. Take 200ul of the peripheral blood sample of the subject to be tested, add 40ul of anti-CD14-FITC (BD company, product number 555397) and 10ul of anti-CD16-APC (BD company, product number 561304), and incubate at room temperature for 15 minutes in the dark;
[0072] 2. Add 200 ul of fixative and incubate at room temperature in the dark for 5 minutes; the components of the fixative are: 1% formaldehyde by volume and 0.35% methanol by volume;
[0073] 3. Add 3 mL of lysate to the sample, vortex to mix, and incubate in the dark for 15 minutes to lyse red blood cells; the lyse contains 10mM Tris-HCL, 10mM NaH 2 PO 4 and / or NaHPO 4 , 130mM NaCl, 1% by volume TritonX-100, 10mM Na 4 P 2 o 7 10H 2 O, pH=7.5;
[0074] 4. Centrifuge at 800g for 5 minutes to remove the supernatant; pour off the supernatant slowly, absorb the residual liquid with paper, and resuspend the cells by vortexing;
[0075] 5. Add 100ul of blocking and punching buffer, and mix with fingers;
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com