Branched polyorganosiloxanes and related curable compositions, methods, uses, and devices
A technology of polyorganosiloxane and silicone, which is applied in semiconductor/solid-state device components, electrical components, coatings, etc., and can solve problems such as increasing curing speed and affecting curing speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
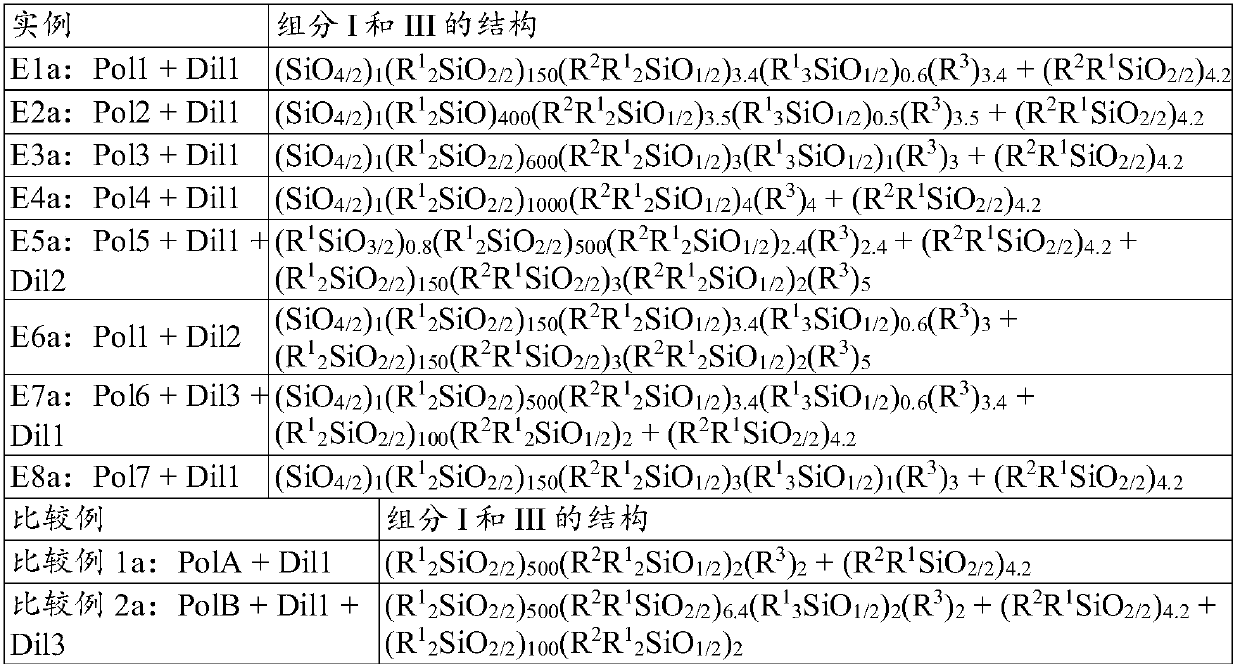
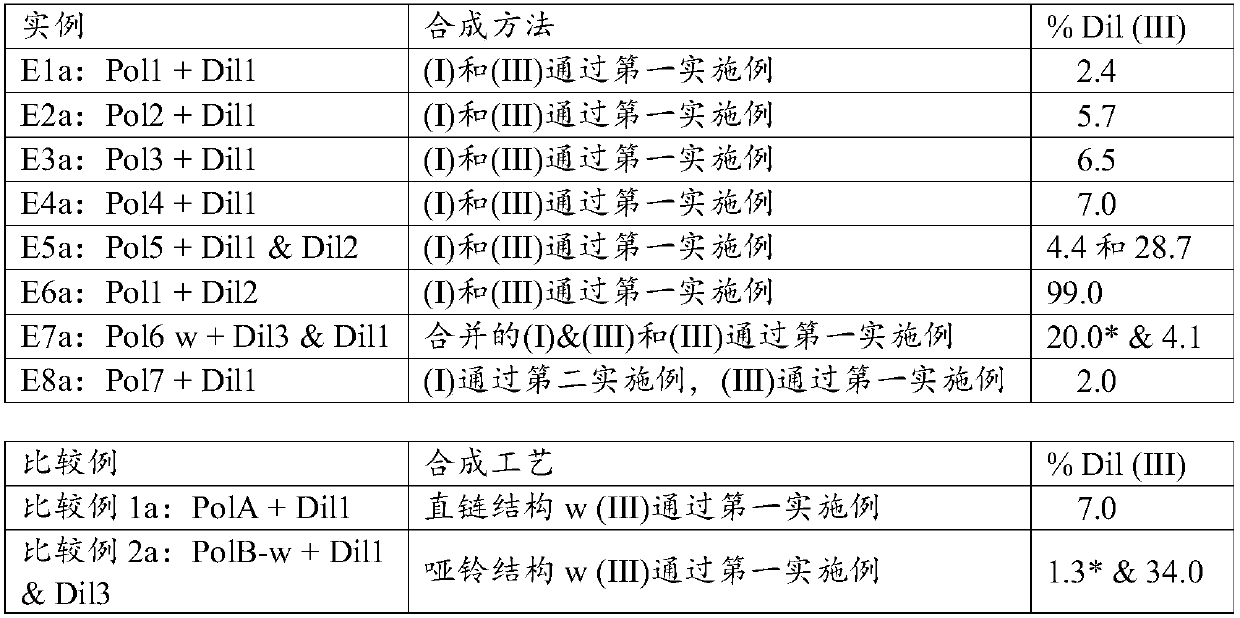
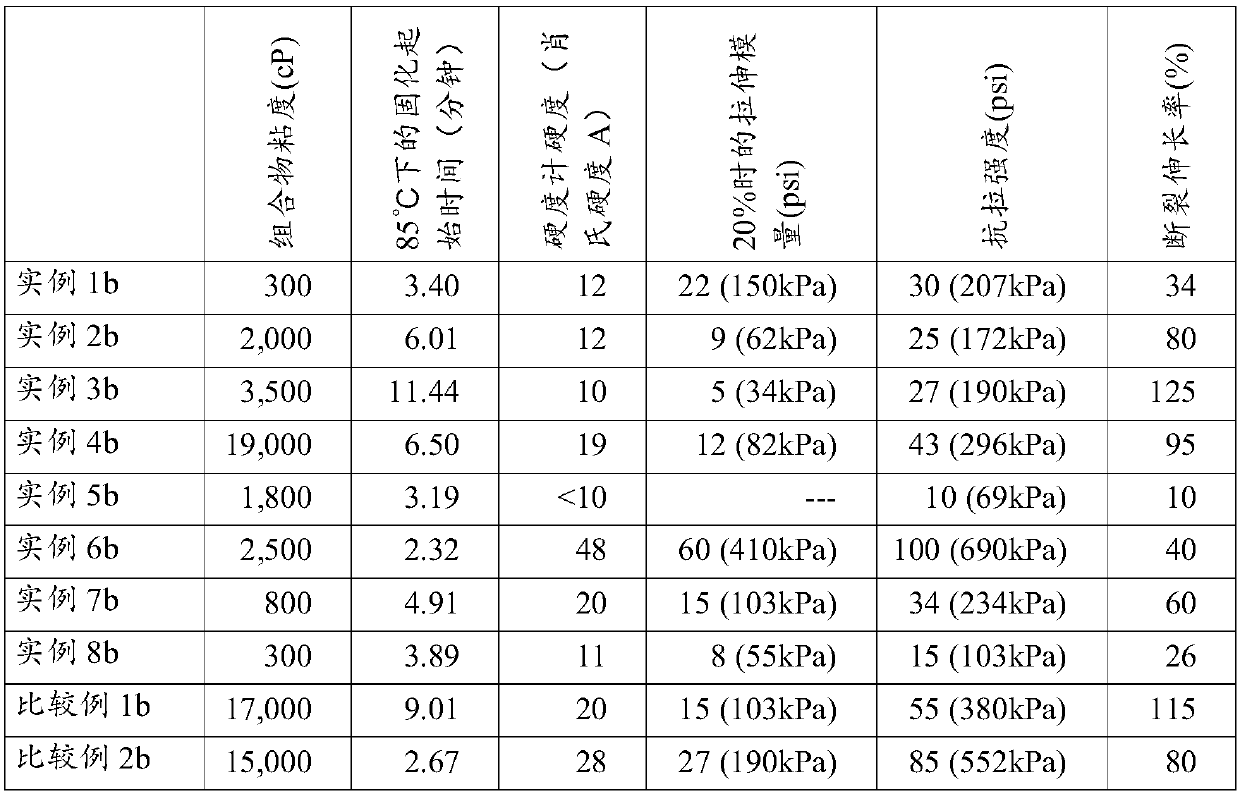
[0174] Several structures were synthesized to provide examples to support the description of the utility of the curable silicone composition. Seven different radically curable branched polyorganosiloxanes of the present invention (I) were synthesized and referred to as Polymers 1 to 7 (or abbreviated as "Pol 1" to "Pol 7"), these polyorganosiloxanes There are various structures consistent with the disclosed structures given herein for the free radically curable branched polyorganosiloxane of component (I). For comparison, two other structures generally known in the art were also synthesized. Comparative Polymer A is a linear polyorganosiloxane with methacrylate functionality at the end. Comparative polymer B is a linear polyorganosiloxane with aggregated methacrylate functional groups at the ends, as disclosed in patent WO 2014124364. The number of reactive groups in a given weight of Polymers 1 to 7 or Comparative Polymers A or B varied and was not consistent. We added an ...
example 1
[0186] Example 1: Preparation of Polymer 1 (Pol1). To prepare the acrylate-functionalized branched polyorganosiloxane (for polymer 1 in Example 1), a mixture of 9 millimoles (mmol) of [SiO 4 / 2 ] 1 [Si(CH 3 ) 2 O] 150 [SiR(CH 3 ) 2 o 1 / 2 ] 4 (where R is approximately 85 mol% vinyl (-CH=CH 2 ) and 15mol% methyl (-CH 3 ) and a blend of 48 mmol of tetramethyldisiloxane and 10 ppm of platinum as a catalyst. A molar ratio of 9 / 48 was set to allow an excess of tetramethyldisiloxane to minimize any chain extension in order to obtain the desired capping reaction. The blend was heated to a temperature of 50° C. and mixed for 30 minutes to allow the hydrosilylation reaction to complete, then stripped at 80° C. and a pressure below 10 Torr (1.3 kPa) for 1 hour. To the resulting residual Si-H functionalized species was added 100 ppm butylated hydroxytoluene, 100 ppm methyltriacetoxysilane and 100 ppm ethyltriacetoxysilane (both from DOW ETS 900) and 4.3 mmol allyl methacrylate...
example 2
[0188] Example 2: Preparation of Polymer 2 (Pol2). Polymer 2 used in Example 2 was prepared similarly to Polymer 1 of Example 1, except that the starting polymer was [SiO 4 / 2 ] 1 [Si(CH 3 ) 2 O] 400 [SiR(CH 3 ) 2 o 1 / 2 ] 4 , where R is about 88.5 mol% vinyl (-CH=CH 2 ) and 12.5mol% of methyl (-CH 3 ) to obtain an example Pol2 of a radically curable branched polyorganosiloxane (I) having the following formula: (SiO 4 / 2 ) 1 (R 1 2 SiO) 400 (R2 R 1 2 SiO 1 / 2 ) 3.5 (R 1 3 SiO 1 / 2 ) 0.5 (R 3 ) 3.5 , where R 1 for CH 3 , R 2 for -Si(CH 3 ) 2 -O-Si(CH 3 ) 2 -(CH 2 ) 3 o 2 CC(CH 3 )=CH 2 and R 3 for CH 2 CH 2 .
[0189] Example 2a: The blend of Example 2a was obtained by blending Pol2 of Example 2 with Dil1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com