Protein marker and method for screening drug anaphylactoid reaction susceptive groups
An allergic reaction and protein labeling technology, applied in the field of molecular biology, can solve the problems of not detecting the risk of anaphylactic reaction in patients in advance, not monitoring anaphylactic reaction, inapplicability, etc., to improve safety, high sensitivity and specificity. , easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The determination of the expression level of characteristic protein markers in normal population includes the following steps:
[0039] 1) Collect peripheral blood samples from 15 test subjects who did not experience clinical anaphylaxis, and collect 1.5-2mL peripheral blood for each sample.
[0040] 2) Use the finished ELASA kit (Human Mas-Related G-Protein Coupled ReceptorMember X2 (MRGPRX2) ELISA Kit) (Cat. No: MBS9333336) method to detect the expression level of MRGPRX2 protein.
[0041] 3) In 15 samples, the maximum value of MRGPRX2 protein expression was 14.43ng / mL, and the minimum value was 12.15ng / mL.
Embodiment 2
[0042] Example 2 Determination of the expression level of characteristic protein markers in allergic populations, including the following steps:
[0043] 1) Collect peripheral blood samples from 15 test subjects who were clinically confirmed to have anaphylactoid reactions, and collect 1.5-2 mL of peripheral blood for each sample.
[0044] 2) Use the finished ELASA kit (Human Mas-Related G-Protein Coupled ReceptorMember X2 (MRGPRX2) ELISA Kit) (Cat. No: MBS9333336) method to detect the expression level of MRGPRX2 protein.
[0045] 3) Among the 15 samples, the maximum value of MRGPRX2 protein expression was 25.65ng / mL, and the minimum value was 20.58ng / mL.
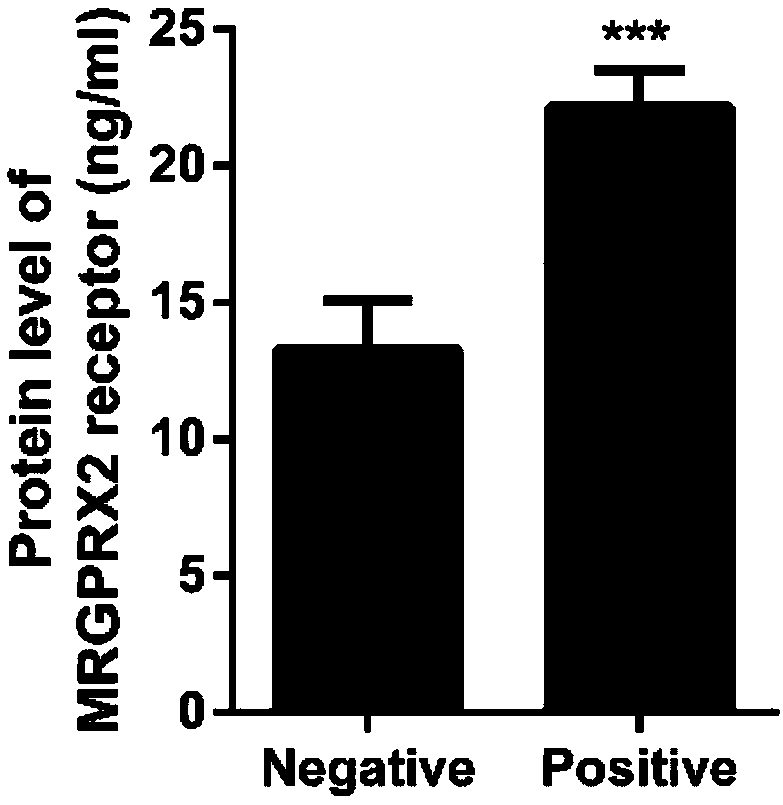
[0046] The measurement results according to Example 1 and Example 2 are shown in figure 2 in, according to figure 2 It can be clearly seen that the expression level of peripheral blood MRGPRX2 protein in the testers with anaphylaxis (Positive) was significantly higher than that in the testers without anaphylaxis (Negati...
Embodiment 3
[0055] Using Western-Blot to verify the expression of the characteristic protein marker MRGPRX2 protein in the allergic population and the normal population, including the following steps:
[0056] 1) Extract the total protein in the blood of allergic people and normal people, and use the BCA protein quantification kit (Bebo Bio BB-3401-500T) to determine the amount of protein loaded.
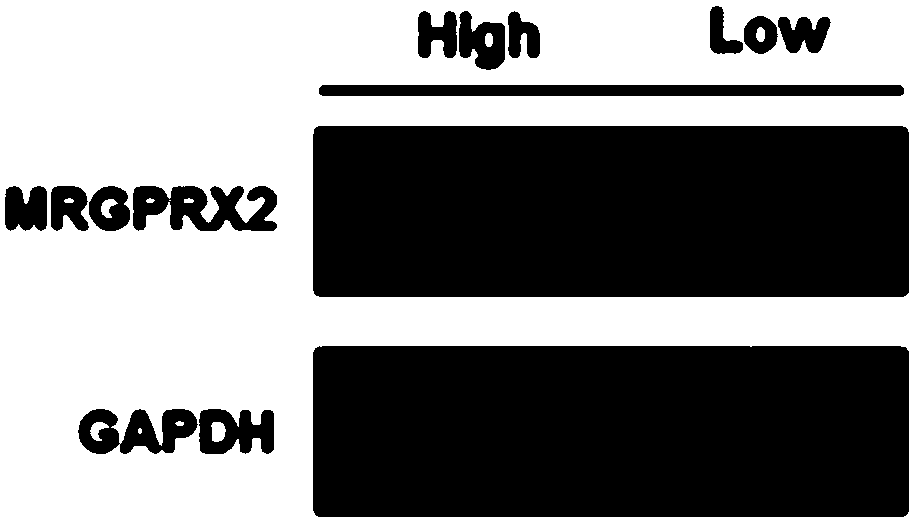
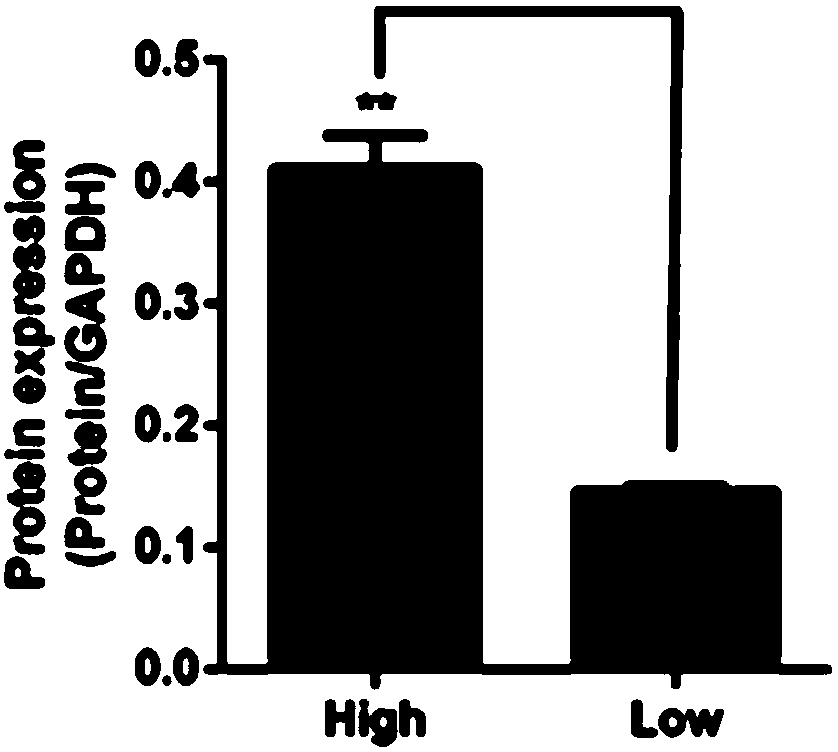
[0057] 2) Western-Blot SDS-PAGE Kit (PIONEER; WBO1) was used to detect the relative expression of MRGPRX2 protein in the allergic population and the normal population. The experimental results are shown in Picture 1-1 with Figure 1-2 in, especially according to Figure 1-2 It can be clearly seen from the results that, compared with the normal population (low), the relative expression of MRGPRX2 protein in the peripheral blood of the drug-like hypersensitivity population (high) is significantly increased, which is consistent with the results of the ELASA experiment.
[0058] Western-Blot was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Expression | aaaaa | aaaaa |
| Expression | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com