Anticancer active peptide and application
A technology of anti-cancer activity and anti-cancer peptides, applied in the direction of peptides, peptide sources, anti-tumor drugs, etc., can solve the problems of weak tumor effect, high toxicity, short half-life of anti-cancer peptides, etc., and achieve strong anti-cancer activity and enhanced effect , the effect of high serum stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
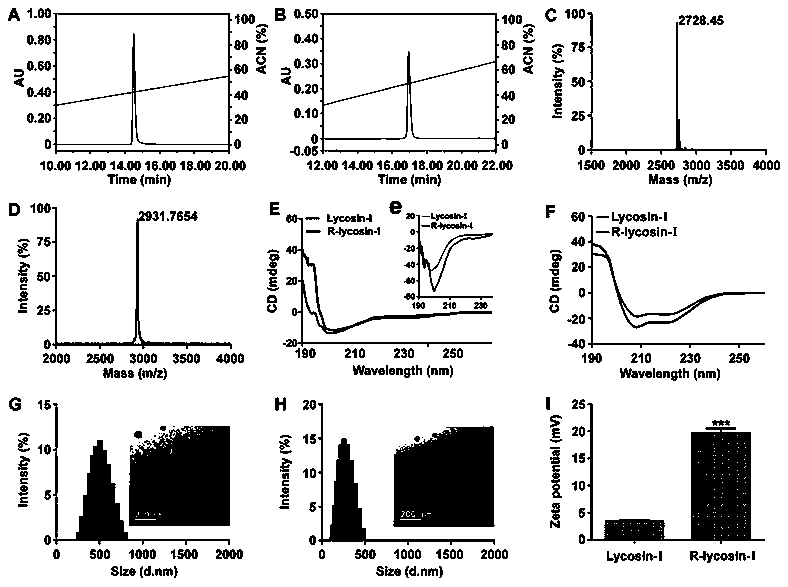
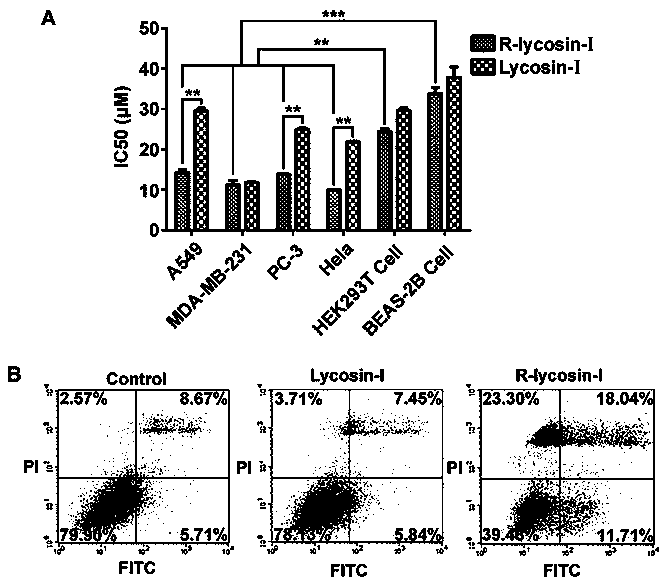
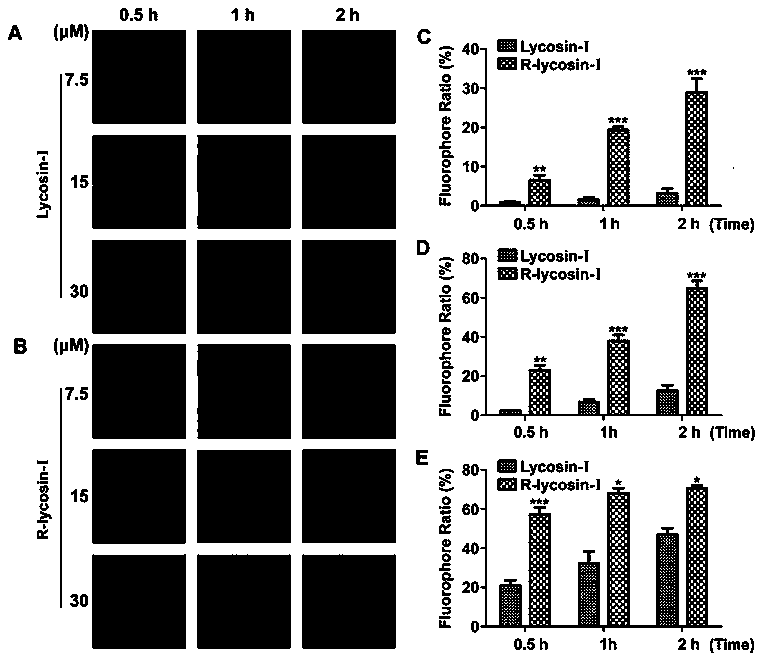
[0026] In order to reveal the anticancer effect and activity enhancement mechanism of R-lycosin-I, we detected the activity of R-lycosin-I on A549 at the cellular level and in 3D tumor spheres, and all experiments used Lycosin-I as a control. All results were statistically and analyzed. The experiments are carried out strictly according to the Institute of Standardization.
[0027] 1. Main materials and instruments
[0028] Reagents related to cell culture were purchased from Thermo Fisher Corporation, and other reagents related to cell experiments were produced by themselves. A549 and H460 cells were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences. Fluorescently labeled Lycosin-I and R-lycossin-I were both from Jill Biochemical Company. Cellometer K2, DLS, TEM, CD, fluorescence microscope are all from our laboratory. Antibody of P27 and cytochrome C was purchased from BD Company.
[0029] 2. Experimental methods and results
[0030] 2.1 Cytotoxi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com