Antipyretic and antiviral active part of a compound traditional Chinese medicine and its preparation method and application
An active part, the technology of Reduning, which is applied to the antipyretic and antiviral active part of the traditional Chinese medicine compound Reduning injection and its preparation field, can solve problems such as potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
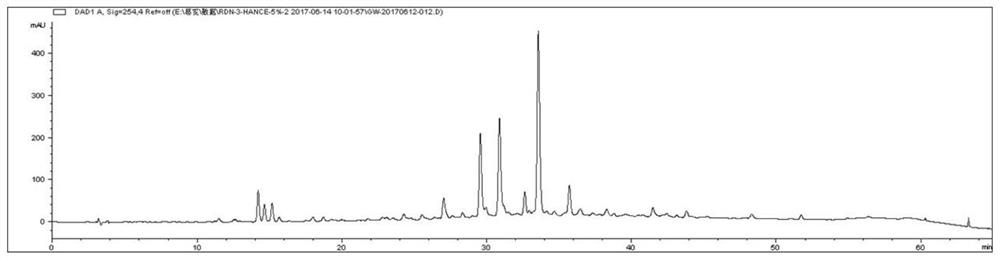
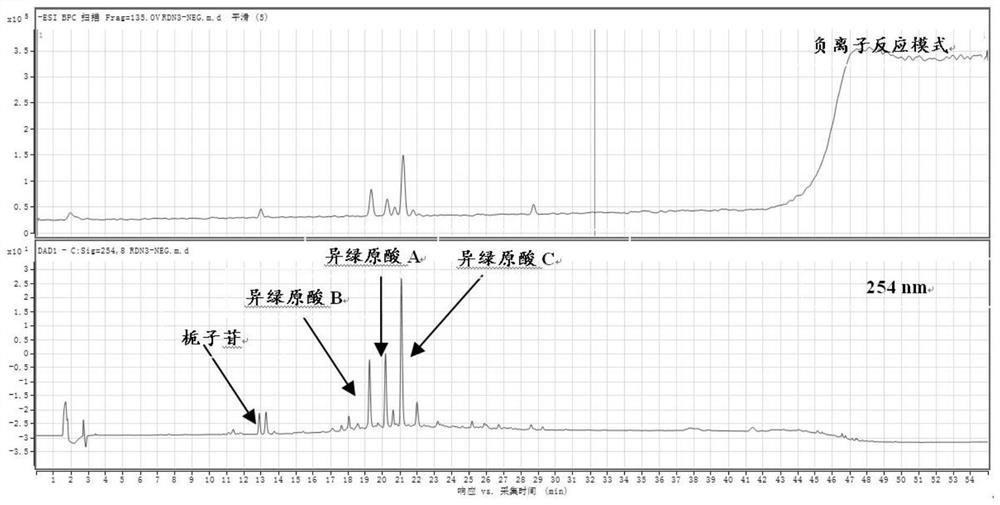
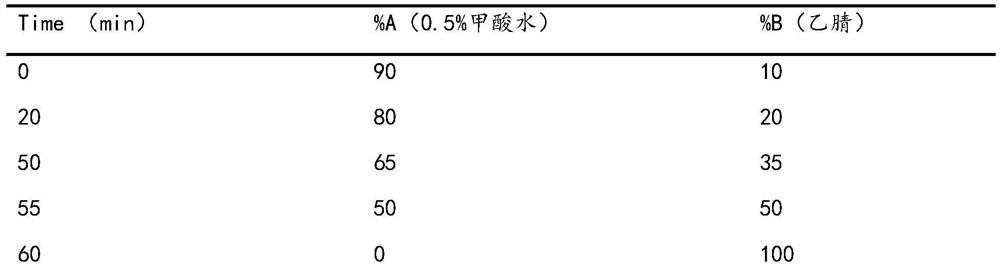
[0026] Take the finished product of Reduning Injection and dry it under pressure to obtain 1 kg of the finished product concentrate, suspend it in 7.5 L of water, and separate it through HP-20 macroporous adsorption resin column chromatography, and then add water, 20%, 30%, 60%, 75% , eluted with 100% ethanol, collected 75%-100% ethanol eluate, concentrated under reduced pressure to no alcohol smell, and obtained 108 g of antipyretic and antiviral active fractions after freeze-drying. Wherein, in terms of mass percentage, the geniposide in the active part is 4.5%, the isochlorogenic acid B is 14.0%, the isochlorogenic acid A is 18.8%, and the isochlorogenic acid C is 32.5%.
preparation example 2
[0028] Take the finished product of Reduning Injection and dry it under pressure to obtain 1 kg of the finished concentrated solution, suspend it in 7.5 L of water, separate it through AB-8 macroporous adsorption resin column chromatography, and then wash it with water, 20%, 30%, 60%, 75% , eluted with 100% ethanol, collected 75%-100% ethanol eluate, concentrated under reduced pressure to no alcohol smell, and obtained 120 g of antipyretic and antiviral active fractions after freeze-drying. Wherein, in terms of mass percentage, the geniposide in the active part is 5.2%, the isochlorogenic acid B is 15.0%, the isochlorogenic acid A is 16.0%, and the isochlorogenic acid C is 28.4%.
preparation example 3
[0030] Take the finished product of Reduning Injection and dry it under pressure to obtain 1 kg of the finished concentrated solution, suspend it in 7.5 L of water, and separate it by chromatography on a DM301 macroporous adsorption resin column. % ethanol for elution, collect 75%-100% ethanol eluate, concentrate under reduced pressure until there is no alcohol smell, and freeze-dry to obtain 112 g of antiviral active fraction. Among them, in terms of mass percentage, the geniposide in the active part is 2.0%, the isochlorogenic acid B is 12.2%, the isochlorogenic acid A is 15.7%, and the isochlorogenic acid C is 27.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com