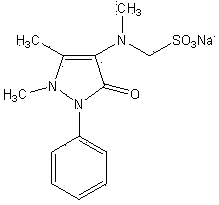
Preparation method for analgesic and antipyretic drug-analgin
Analgin, a synthetic method technology, applied in the field of drug synthesis, can solve the problems of polluting the environment, low yield, etc., and achieve great economic benefits and reduce environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
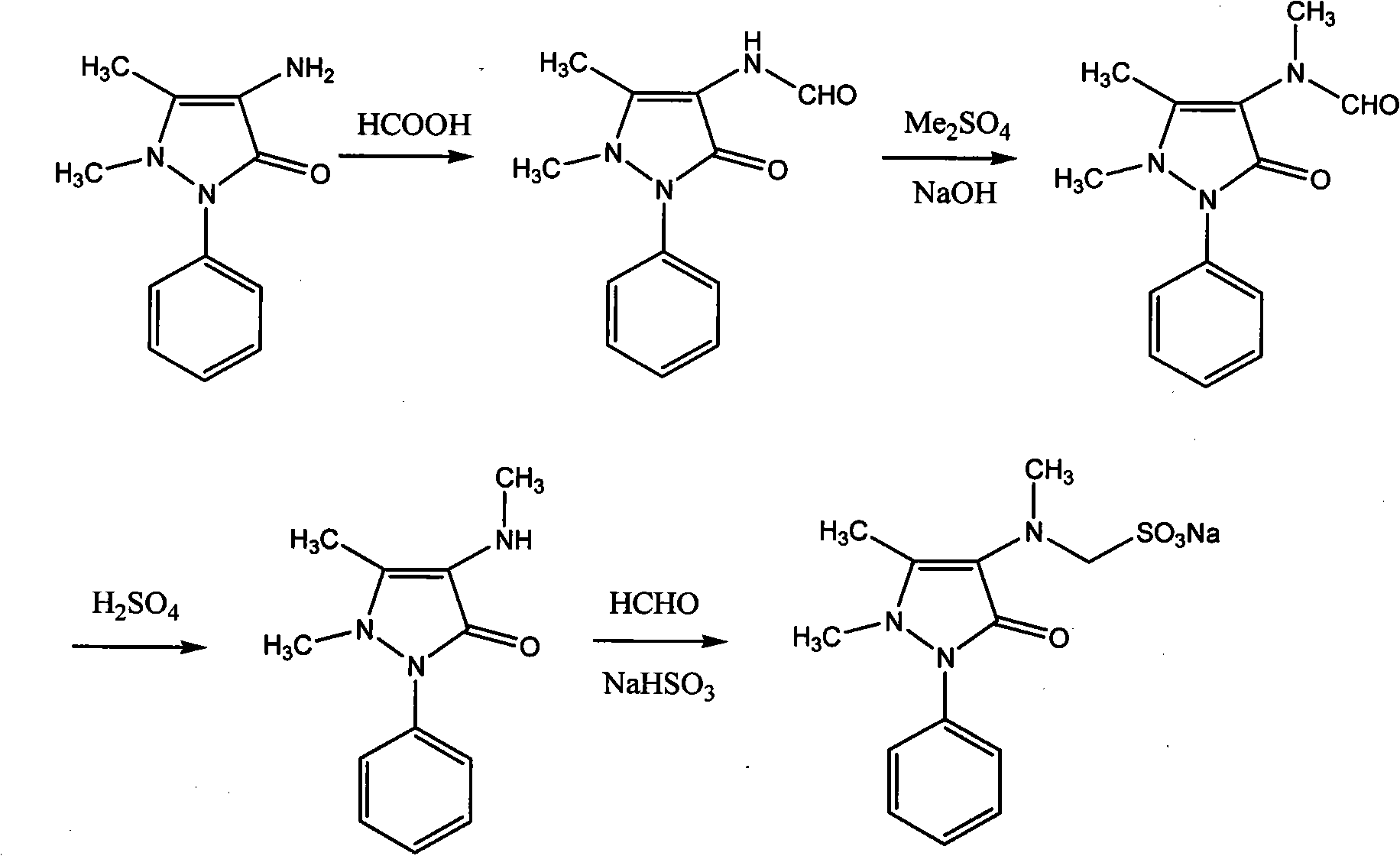
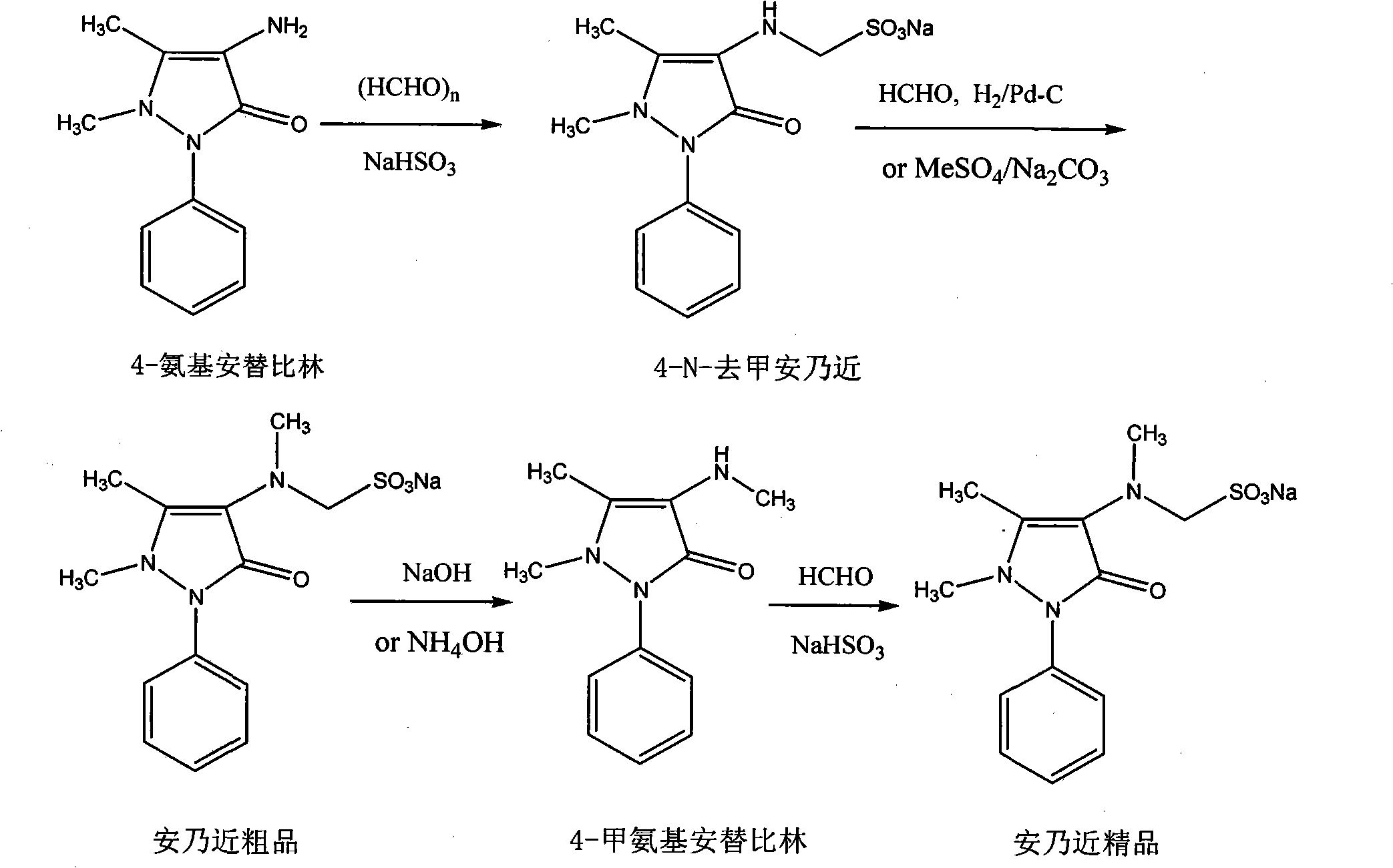
[0049] Example 1 Preparation of 4-N-desmethyl Sulpyrine
[0050] 20.3 g of 4-aminoantipyrine (AA) (0.1 mol), 10.4 g of sodium bisulfite (0.1 mol), 3 g of paraformaldehyde (0.1 mol) and 100 ml of 95% ethanol were added to a 250 ml reaction bottle, stirred and heated to reflux for 1 hour. After the reaction liquid was lowered to 45°C, solid 4-N-desmethyl sulpyrine was added to the reaction liquid to cause crystallization. After vigorously stirring for half an hour, the solid was collected by filtration to obtain 30.3 g of 4-N-desmethyl sulpyrine. The rate is 90%; melting point: 217°C; 1 H NMR (400MHz, DMSO-d 6 )δ: 2.18(s, 3H), 2.80(s, 3H), 3.78(d, J=6.96Hz, 2H), 4.00(m, 1H), 7.25(m, 1H), 7.45(m, 4H); 13CNMR (100MHz, DMSO-d 6 ) δ 161.8, 140.8, 135.4, 128.9(2), 125.3, 122.1(2), 120.0, 62.4, 37.7, 10.4.
Embodiment 2
[0051] Embodiment 2 The preparation of Sulpyrine crude product
[0052] 33 grams of 4-N-desmethyl sulpyrine (0.1mol) was dissolved in 100 milliliters of 95% ethanol, and 10 milliliters of 35% formaldehyde aqueous solution (0.13mol), 2 grams of 10% palladium carbon and 5 milliliters of pH6. 86 phosphate standard buffer solution (prepared by sodium dihydrogen phosphate and dipotassium hydrogen phosphate), vacuumize after transferring to the hydrogenation tank, then feed hydrogen, start stirring, and keep the hydrogen pressure at 0.1MPa to react for two hours, and the reaction temperature is controlled At 60-85° C., after the reaction was completed, the filtrate was heated and filtered, and the filtrate crystallized after cooling, and the solid was collected by filtration to obtain 34.5 g of a crude product of Sulpyrine, with a yield of 98%.
Embodiment 3
[0053] The preparation of embodiment 3 4-methylaminoantipyrine (MAA)
[0054] 33 gram Sulpyrine crude products (0.1mol) are dissolved in 50 milliliters of water, then add 5 milliliters of 10% sodium hydroxide, be heated to 50 ℃ of reaction 1 hour, separate after cooling and obtain MAA21.6 grams (yield 98% ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com