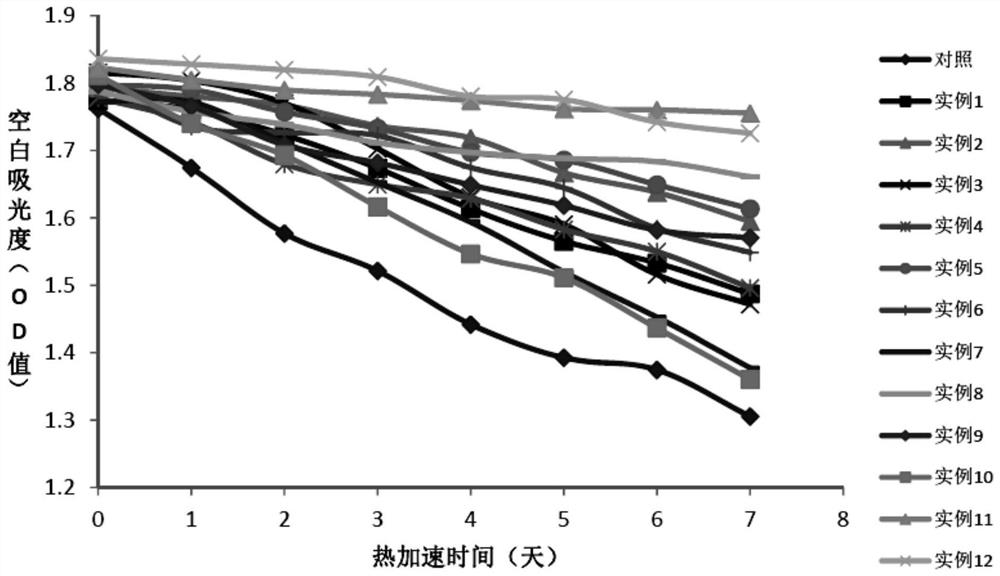
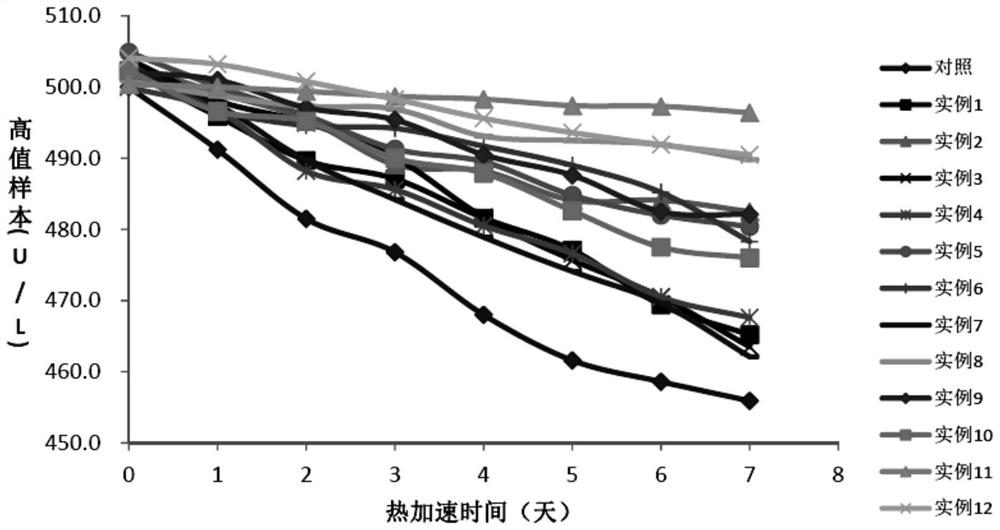
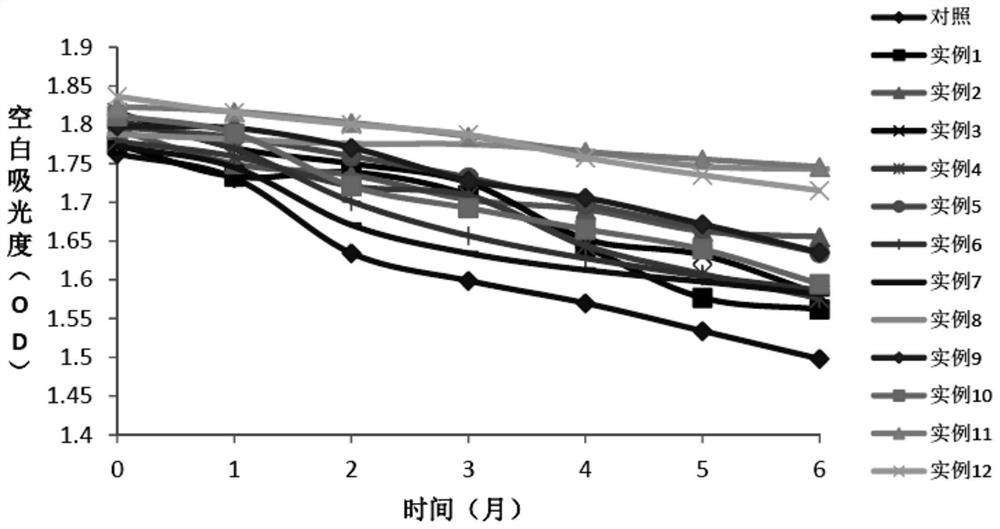
A stable reagent for the determination of aspartate aminotransferase
A technology of aspartate aminotransferase and reagents, which is applied in the field of biochemistry and clinical testing, and can solve problems such as reagent instability, difficulty in detecting serum AST, and accelerated stability experiments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: the preparation of comparison kit
[0065] First Reagent R1:
[0066]
[0067] Second reagent R2:
[0068] TRIS, pH6.5 100mmol / L
[0069] L-Aspartic Acid 720mmol / L
[0070] α-ketoglutarate 36mmol / L
Embodiment 2
[0071] Embodiment 2: the preparation of test kit
[0072] The preparation method of the second reagent R2 is the same as in Example 1; the preparation method of the first reagent R1 is shown in Table 1.
[0073] Table 1. R1 reagent composition of different test kits
[0074]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com