Application of Ruboxistaurin in preparation of medicine for preventing and treating pulmonary fibrosis and hepatic cirrhosis and medicinal preparation thereof
A pulmonary fibrosis and drug technology, applied in drug combination, digestive system, medical formula, etc., can solve problems such as unsatisfactory curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
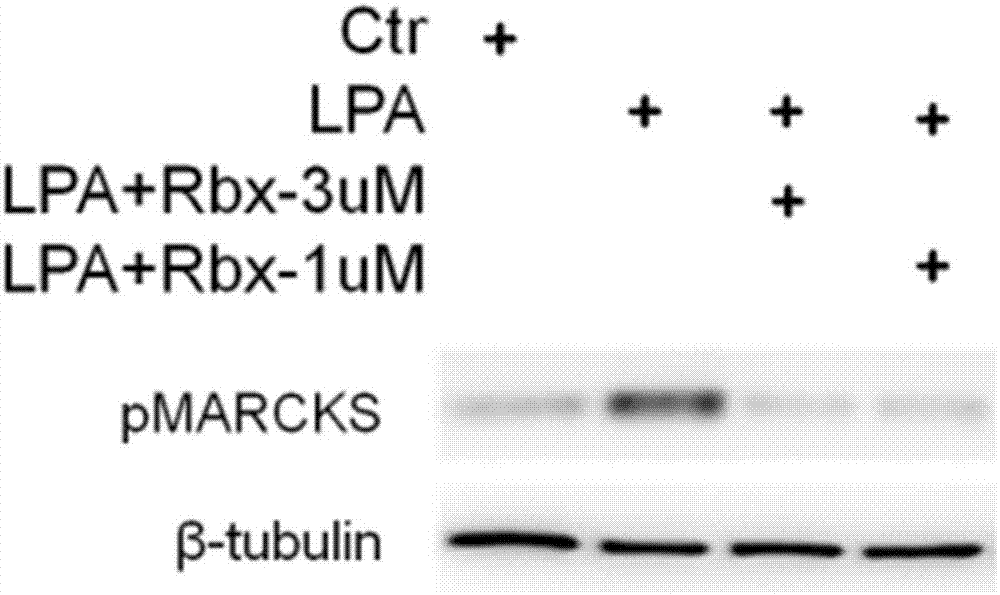
[0019] Example 1: Ruboxistaurin inhibits the migration of mouse fibroblasts (in vitro fibrosis markers) experiment
[0020] In the Transwell cell migration assay, mouse fibroblasts were starved overnight in serum-free culture, and then the cells were resuspended in DMEM medium containing 0.1% fetal bovine serum, and seeded into the upper chamber of the Transwell of a 24-well plate, and inoculated in each well 5×104 cells. Add 0.1% fetal bovine serum to the DMEM medium in the lower chamber and figure 1 Indicates the inclusion or absence of lysophosphatidic acid (LPA, lysophosphatidic acid) or Ruboxistaurin (Rbx). There are 4 cell treatment methods (from left to right in the upper picture): the first one, adding DMEM medium containing 0.1% fetal bovine serum to the lower chamber of Transwell; the second method, adding 0.1% fetal bovine serum to the lower chamber of Transwell and DMEM medium of 5 μM LPA; the third type, the DMEM medium containing 0.1% fetal bovine serum, 5 μM L...
Embodiment 2
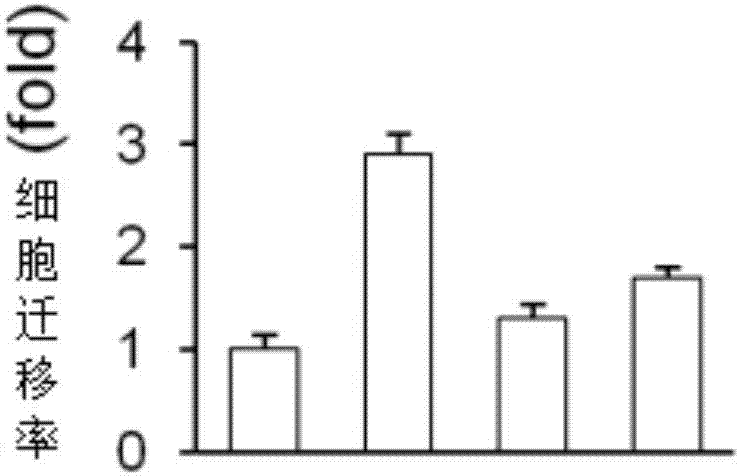
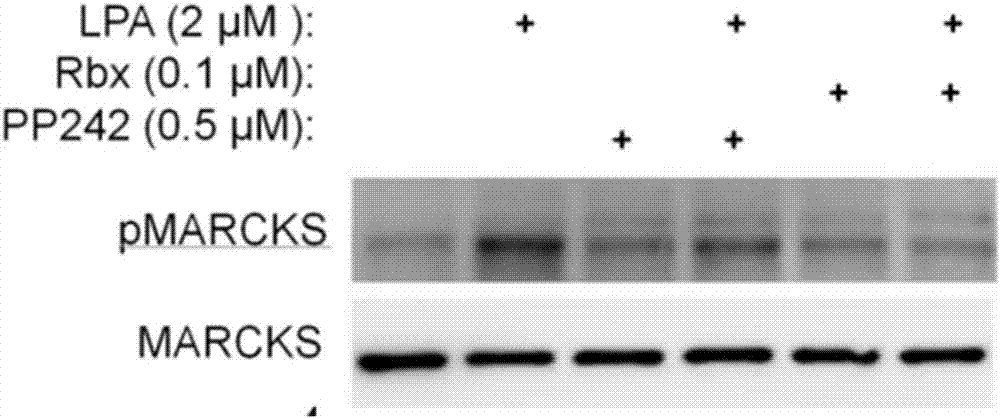
[0022] Example 2: Ruboxistaurin inhibits migration of human fibroblast cell line (LL29) derived from patients with idiopathic pulmonary fibrosis (IPF)
[0023] In the Transwell cell migration assay, LL29 cells were starved overnight in serum-free culture, and then the cells were resuspended in DMEM medium containing 0.1% fetal calf serum, and seeded into the upper chamber of the Transwell of a 24-well plate, with 5×10 cells per well. 4 cell. In the lower chamber, DMEM medium with 0.1% fetal bovine serum was added with or without LPA, PP242 (an mTOR inhibitor) or Ruboxistaurin (Rbx) as shown in the upper figure. 6 cell processing methods ( Figure 4 From left to right) are: the first one, DMEM medium containing 0.1% fetal bovine serum was added to the lower chamber of Transwell; the second one was cultured in DMEM containing 0.1% fetal bovine serum and 2 μM LPA in the lower chamber of Transwell base; the third type, the DMEM medium containing 0.1% fetal bovine serum and 0.5 μ...
Embodiment 3
[0025] Example 3: Delayed induction of the role of Ruboxistaurin in the formation of pulmonary fibrosis in the model of pulmonary fibrosis in mice
[0026] (1) The reagents used in the experiment were prepared as follows:
[0027] Preparation of Ruboxistaurin solution and blank control:
[0028] 1. Prepare a 0.8% water-soluble hydroxyethylcellulose 250HHX solution (Natrosol, 250HHX) with purified water;
[0029] 2. Add Tween80 to the above polymer solution, the final concentration of Tween80 is 0.5%;
[0030] 3. Add a solid sample of Ruboxistaurin to the above polymer solution at a concentration of 0.5 mg / ml, and stir evenly.
[0031] The above solution is prepared before use and should be used within one hour.
[0032] (2) The experiment operates as follows:
[0033] 1. 10-week-old C57BL / 6 mice were instilled with 50 μl of bleomycin by nasal feeding every day at a dose of 2.5 mg / kg to induce pulmonary fibrosis.
[0034] 2. After 7 days of instillation, the mice were divi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com