Small-molecule inhibitor SLD1338 and application thereof in pharmacy
A technology of small molecule inhibitors and drugs, applied in drug combinations, antineoplastic drugs, organic chemistry, etc., can solve the problems of insignificant effect, little effect, and low toxicity effect in advanced breast cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] A small molecule inhibitor SLD1338, characterized in that the structural formula of the small molecule inhibitor is:
[0037]
[0038] Pharmacophore results
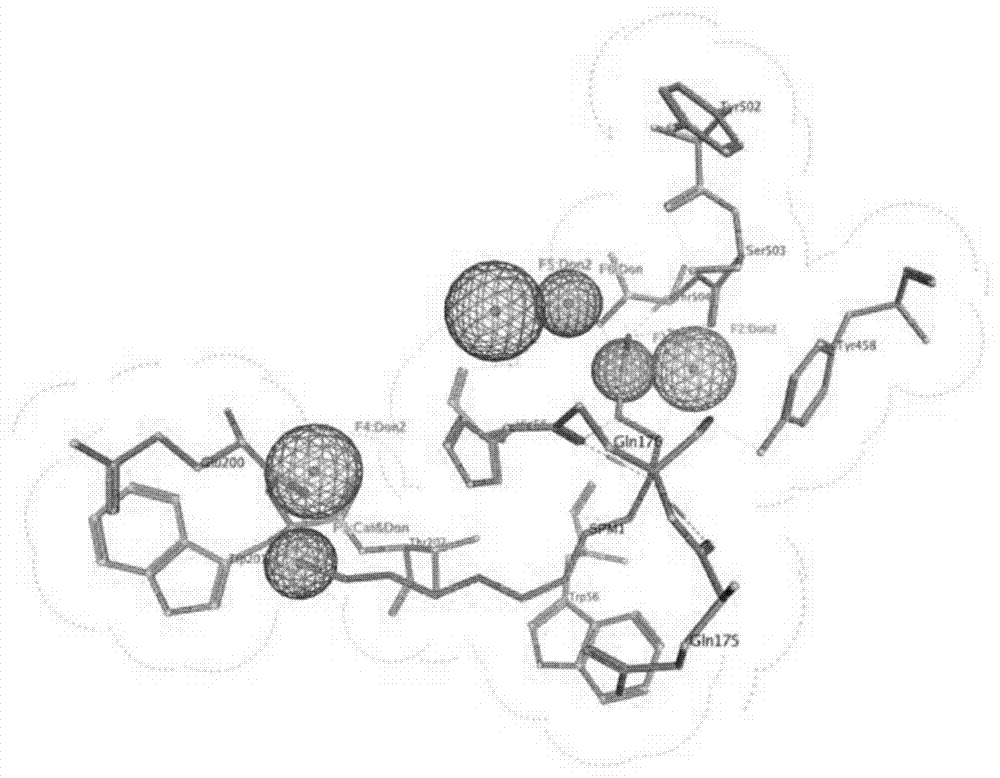
[0039] In the complex structure, a lot of hydrogen bond interactions are formed between Spd and SMO. In order to ensure the rationality of the pharmacophore model, we only kept two pairs of key hydrogen bond characteristic elements. In addition, in order to ensure the diversity of the structure, after analyzing the characteristics of the active pocket, we added a set of hydrogen bond characteristic elements based on acceptor amino acids P1-Ser O. details as follows( figure 1 ): two donor centers - P1-Ser O and P3-Glu O.
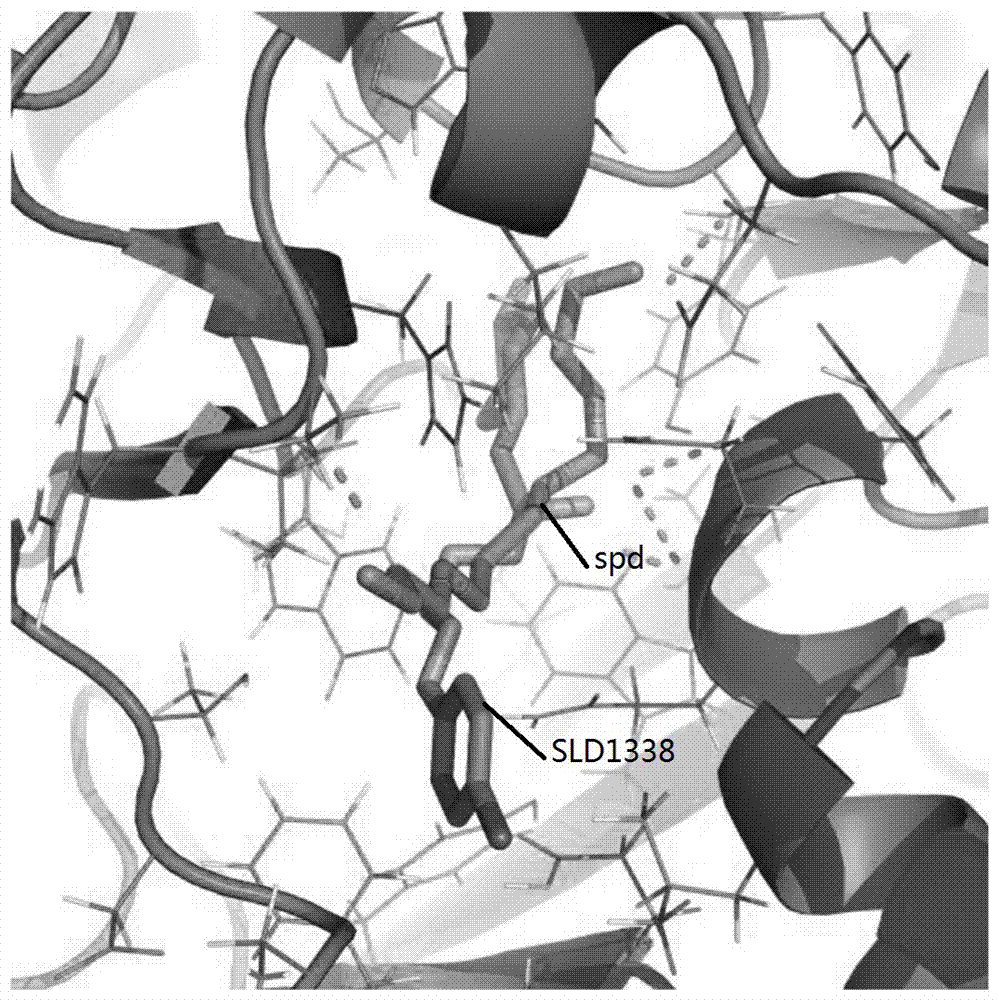
[0040] Molecular docking results
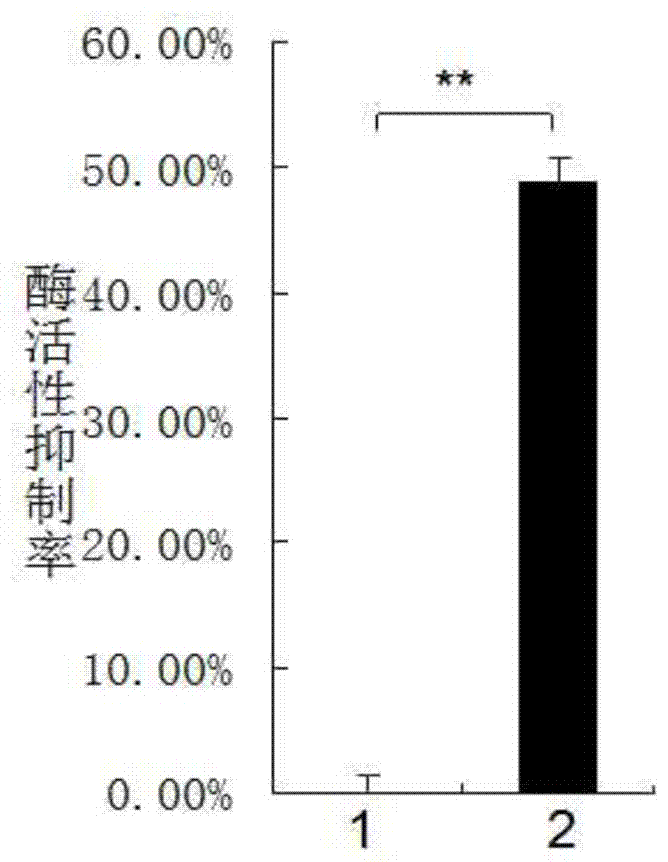
[0041] The compounds obtained through the above pharmacophore model search are optimized, and the optimized compounds are used in the next step of molecular docking screening, and are sorted according to the docking energy scoring. The selected 120 compo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com