Efficient preparation method of polysubstitution phenazine derivative and oxide thereof
A technology of derivatives and oxides, which is applied in the field of efficient preparation of multi-substituted phenazine derivatives and their oxides, can solve problems such as poor preparation and complexity, and achieve simple and easy-to-operate synthesis process, easy-to-use products, and high-efficiency preparation methods. Scientific and reasonable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of 3-methylphenazine-5-oxide
[0044]
[0045] Method 1: Weigh di-p-tolyliodonium trifluoromethanesulfonate (1mmol, 458mg), benzofurazan (1mmol, 120mg), cuprous bromide (0.1mmol, 14.2mg) in a 25mL sealed tube, Add magneton, replace with high-purity nitrogen three times, add 5mL dichloroethane to the sealed tube under nitrogen protection, tighten the sealed tube, move it into an oil bath at 70°C and stir, and react for 12 hours. The reaction was followed by TLC detection, and after the reaction was completed, the sealed tube was cooled to room temperature. Add 5 mL of distilled water to the system to quench the reaction, stir; extract 3 times with 5 mL of dichloromethane, combine the organic phases, add magnesium sulfate to dry, and remove the solvent with a rotary evaporator to obtain a crude product; the crude product is loaded on silica gel and eluted The solvent was purified by column chromatography using petroleum ether:ethyl acetate=10:1 i...
Embodiment 2
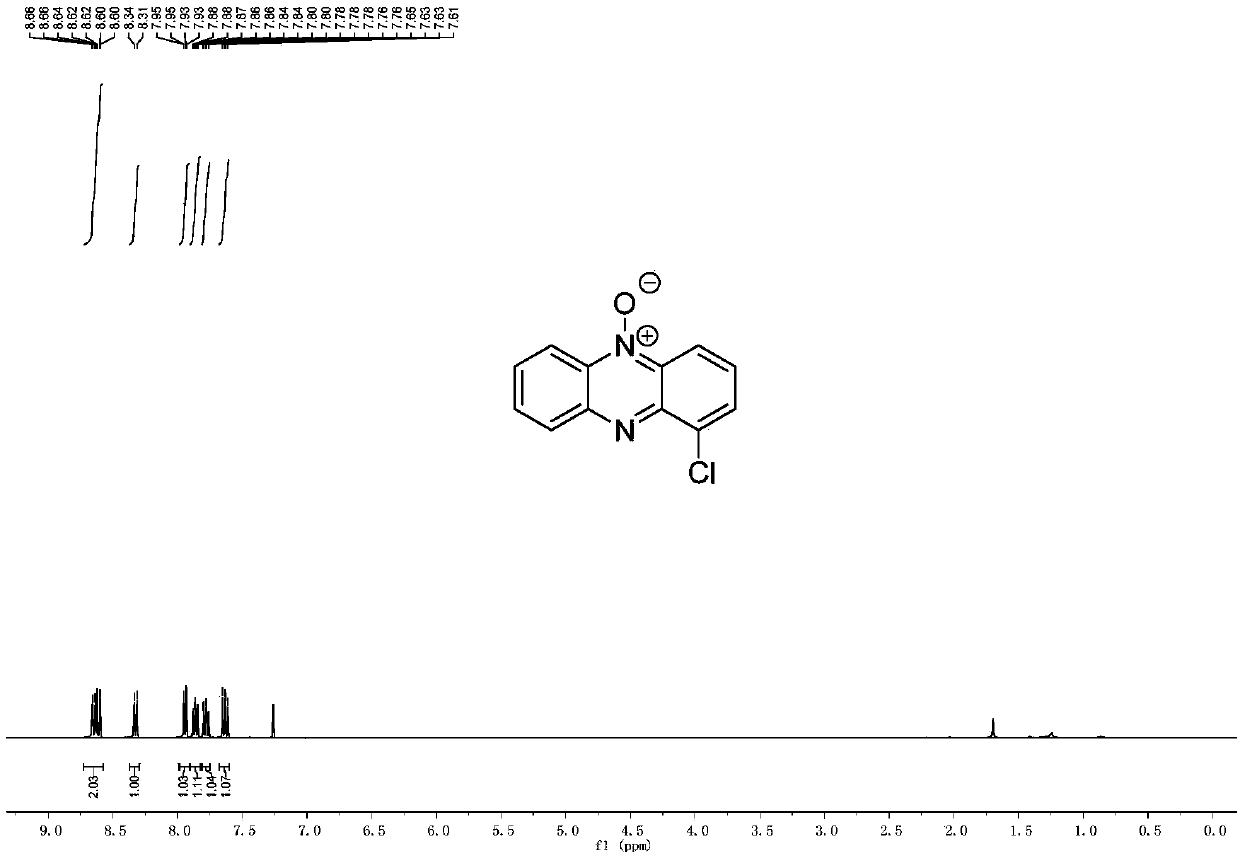
[0058] Example 2 Preparation of 1-chlorophenazine-5-oxide
[0059]
[0060] Method: Weigh di-2-chlorophenyliodonium trifluoromethanesulfonate (1mmol, 458mg), benzofurazan (1mmol, 120mg), cuprous bromide (0.06mmol, 8.52mg) in a 25mL sealed tube Add magnetons, replace with high-purity nitrogen for three times, add 5mL of dichloroethane to the sealed tube under nitrogen protection, tighten the sealed tube, move it into an oil bath at 70°C and stir, and react for 15 hours. The reaction was followed by TLC detection, and after the reaction was completed, the sealed tube was cooled to room temperature. Add 5 mL of distilled water to the system to quench the reaction, stir; extract 3 times with 5 mL of dichloromethane. The organic phases were combined, dried by adding magnesium sulfate, and the solvent was removed by a rotary evaporator to obtain a crude product; the crude product was loaded on silica gel, and the eluent was purified by column chromatography with a volume ratio o...
Embodiment 3
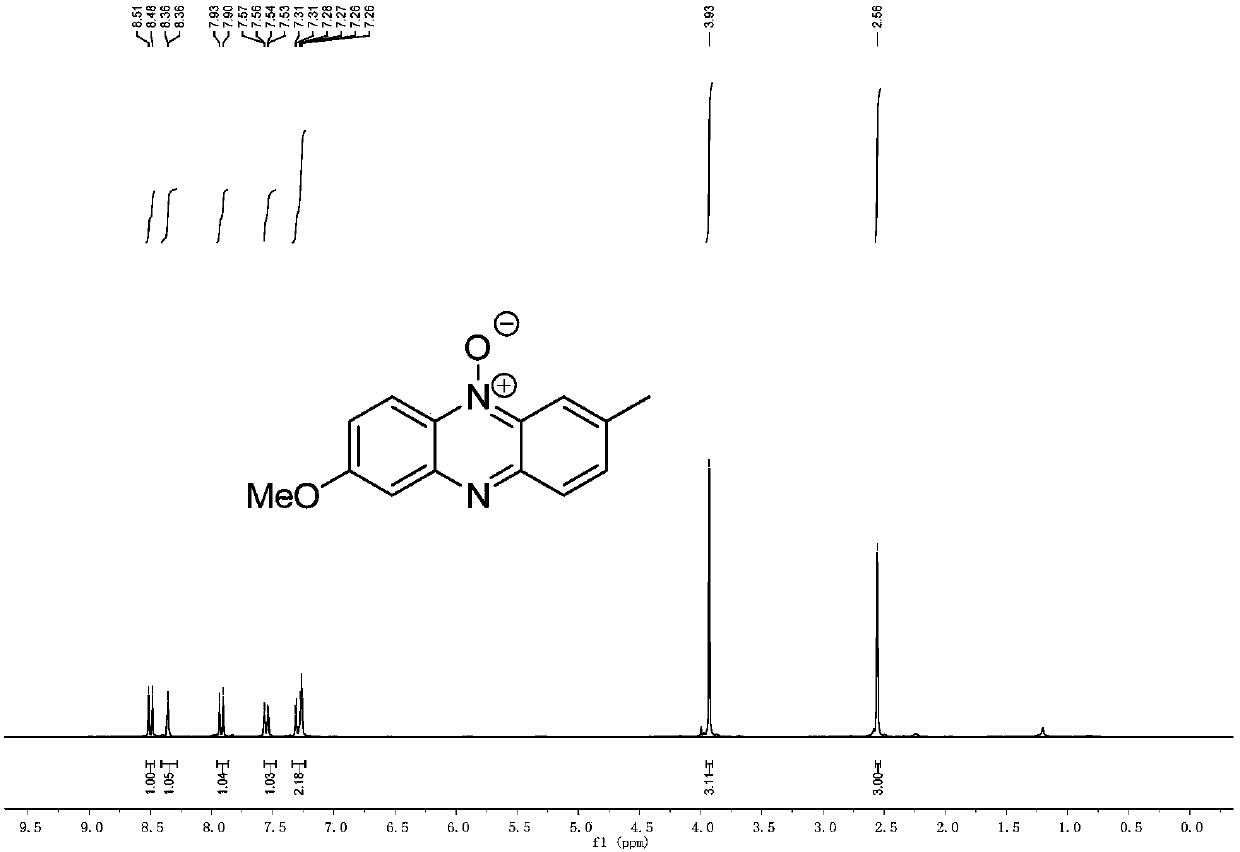
[0068] Example 3 Preparation of 2-methoxy-7-methylphenazine-5-oxide
[0069]
[0070] Method: Weigh di-p-tolyliodonium trifluoromethanesulfonate (1mmol, 458mg), 5-methoxybenzofurazan (1mmol, 150mg), cuprous bromide (0.15mmol, 21.3mg) in 25mL Add magnetons to the sealed tube, replace it with high-purity nitrogen three times, add 7mL dichloroethane to the sealed tube under nitrogen protection, tighten the sealed tube, move it into an oil bath at 80°C and stir, and react for 20 hours . The reaction was followed by TLC detection, and after the reaction was completed, the sealed tube was cooled to room temperature. Add 5 mL of distilled water to the system to quench the reaction, stir; extract 3 times with 5 mL of dichloromethane. The organic phases were combined, dried by adding magnesium sulfate, and the solvent was removed by a rotary evaporator to obtain a crude product; the crude product was loaded on silica gel, and the eluent was purified by column chromatography with a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap