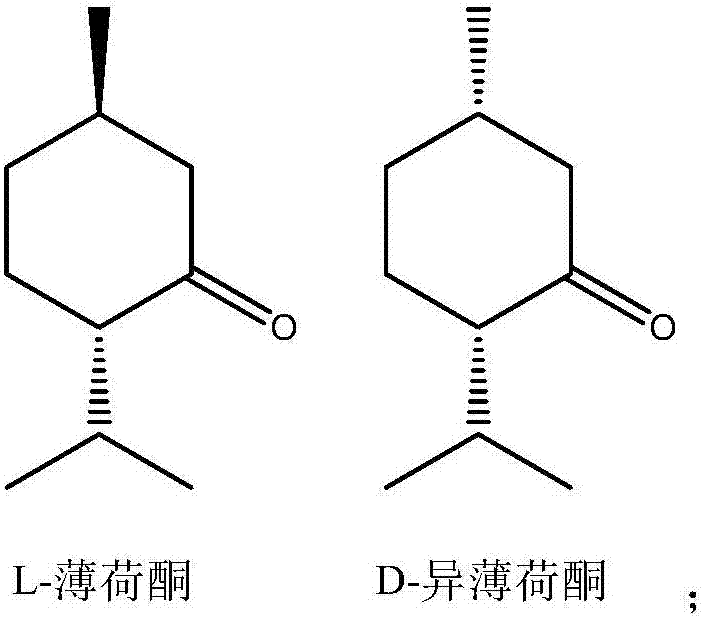
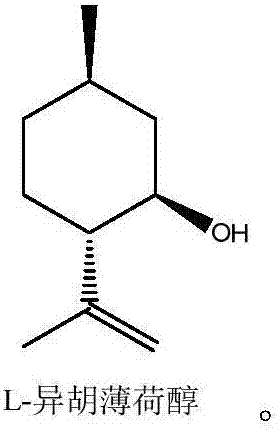
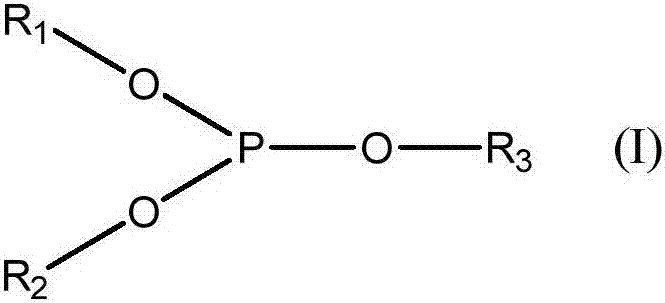
Method for synthesizing chiral menthone enantiomer L-menthone and D-menthone by using L-isopulegol
A kind of technology of isopulegol and isomenthone, applied in the field of preparation of chiral menthone, can solve the problems such as limited turnover number, short catalyst life, unfavorable influence of processing environment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Without taking special precautions, 3.02 mg of iridium trichloride, 0.31 mg of triphenyl phosphite, 2.46 mg of DBU phenate, and 162.37 g of L-isopulegol (95 wt %) were transferred to the reaction vessel and stirred until mixed Liquid is clear and transparent. The absolute pressure of the reaction was maintained at 0.2 MPa by feeding high-purity nitrogen gas, and the mixture was heated to 120° C. and stirred for 2 hours. Conversion and menthone yield were determined by calibrated GC analysis. The results are summarized in Table 1.
Embodiment 2
[0044] Without taking special precautions, transfer 0.98 mg of iridium acetylacetonate, 3.10 mg of triphenyl phosphite, 4.43 mg of DBU phenate and 173.14 g of L-isopulegol (98wt%) to the reaction kettle, and stir until the mixture Clear and transparent. The absolute pressure of the reaction was maintained at 0.5 MPa by feeding high-purity nitrogen, and the mixture was heated to 130° C. and stirred for 5 hours. Conversion and menthone yield were determined by calibrated GC analysis. The results are summarized in Table 1.
Embodiment 3
[0046] Without special precautions, 67.2 mg of 1,5-cyclooctadiene iridium(I) chloride dimer, 65.16 mg of triphenyl phosphite, 59.12 mg of DBU phenate, and 186.97 g of L-isopulegol (99wt%) was transferred to the reactor, stirred until the mixed solution was clear and transparent. The absolute pressure of the reaction was maintained at 1.0 MPa by feeding high-purity nitrogen gas, and the mixture was heated to 140° C. and stirred for 10 hours. Conversion and menthone yield were determined by calibrated GC analysis. The results are summarized in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com