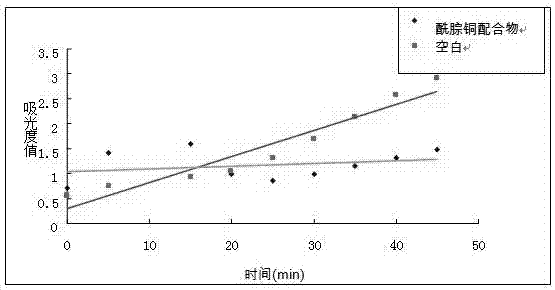
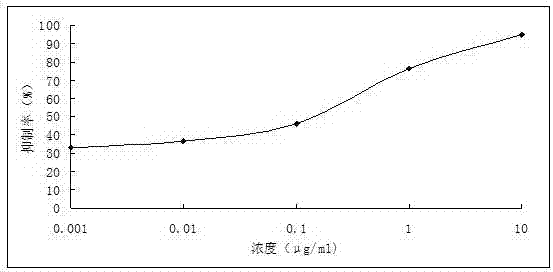
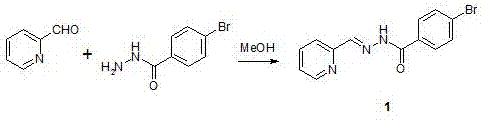Acylhydrazone copper (II) complex and preparation method and application in inhibiting urease activity thereof
A technology of copper acylhydrazone and complexes, which is applied in the field of copper acylhydrazone complexes and their preparation, can solve the problems of toxic and side effects, difficulty in popularization and application, and achieves the effects of good stability, mild experimental environment and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0036] A detailed embodiment of the present invention is as follows:
[0037] Step 1: Dissolve 10.7 g (0.1 mol) of pyridine-2-carbaldehyde and 21.5 g (0.1 mol) of 4-bromobenzoylhydrazide in a round-bottomed flask under stirring at 20±10 ˚C in 100 mL of anhydrous methanol, then mixed and stirred at 20±10 °C, TLC followed the reaction, after 1 h of reaction, the solvent was distilled off under reduced pressure, the obtained solid crude product was washed with cold methanol, dried, and the obtained solid The crude product was dissolved in methanol and recrystallized to obtain a colorless crystalline compound with the structure shown in Formula 1. Yield 95%. 1 H NMR (DMSO- d 6 , 500 MHz) δ (ppm): 8.62 (d, 1H, Py H ), 8.48 (m, 1H,Py H ), 7.95 (d, 2H, Ar H ), 7.83 (m, 3H, Py H and Ar H ), 7.65 (t, 1H, Py H ), 7.43(s, 1H, C H =N), 4.06 (s, 1H, N H ). 13 C NMR (DMSO- d 6 , 300 MHz) δ IR data (KBr, cm -1 ): 3230, 1654, 1589, 1545, 1466, 1432, 1323, 1305, 1279, 114...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com