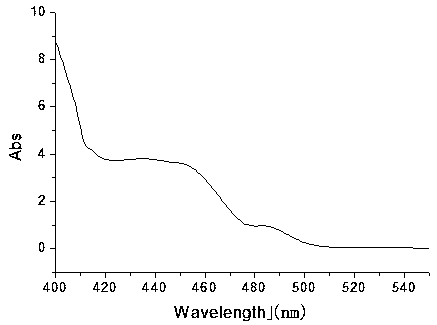
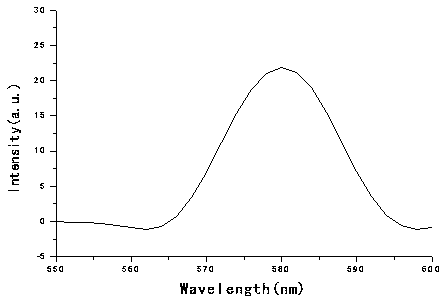
Yellow polyhydroxy phosphorescence iridium complex and preparation method thereof
A technology of phosphorescent iridium complexes and polyhydroxy yellow, which is applied in the fields of indium organic compounds, platinum group organic compounds, chemical instruments and methods, etc., can solve the problems that the complexes have not been reported, and achieve the reduction of concentration quenching effect and the enhancement of anti-allergic effects Effect of response, anti-aging effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: a kind of preparation method of polyhydroxy yellow phosphorescent iridium complex, concrete steps are as follows:
[0025] (1) Synthesis of iridium dichloro bridge: In a round-bottomed three-neck flask, dissolve 2.83g of 2-phenylpyridine and 2.36g of iridium trichloride hydrate into mixed solvent A, wherein 2-phenylpyridine and trichlorohydrin The molar ratio of iridium is 2.6:1, the mixed solvent A is a mixture of 20mL deionized water and 80mL ethylene glycol ether, under the conditions of protective gas nitrogen atmosphere, magnetic stirring (stirring speed 300r / min), and temperature 120°C Reflux for 24 hours, filter to obtain filtrate A and solid A, wash the solid three times with deionized water, ethanol, and acetone in turn, then dissolve in dichloromethane to remove impurities, filter to obtain filtrate B and solid B, and concentrate and dry the filtrate in vacuo The obtained iridium dichloro bridge complex, mass spectrometry (MS / ESI + ) is 1075, an...
Embodiment 2
[0039] Embodiment 2: a kind of preparation method of polyhydroxy yellow phosphorescent iridium complex, concrete steps are as follows:
[0040](1) Synthesis of iridium dichloro bridge: In a three-necked round-bottomed flask, dissolve 1.87g 2-phenylpyridine and 2.12g iridium trichloride hydrate into mixed solvent A, wherein 2-phenylpyridine and trichlorohydrin The molar ratio of iridium chloride is 2:1, the mixed solvent A is a mixture of 30mL deionized water and 70mL ethylene glycol ether, under the conditions of protective gas nitrogen atmosphere, magnetic stirring (stirring speed 350r / min), and temperature 130°C Reflux for 12 hours, filter to obtain filtrate A and solid A, wash the solid with deionized water, ethanol, and acetone 5 times in turn, then dissolve in dichloromethane to remove impurities, filter to obtain filtrate B and solid B, and concentrate and dry the filtrate in vacuo The obtained iridium dichloro bridge complex, mass spectrometry (MS / ESI + ) is 1075, and ...
Embodiment 3
[0044] Embodiment 3: a kind of preparation method of polyhydroxy yellow phosphorescent iridium complex, concrete steps are as follows:
[0045] (1) Synthesis of iridium dichloro bridge: In a three-necked round-bottomed flask, dissolve 2.5g of 2-phenylpyridine and 2.47g of iridium trichloride hydrate into mixed solvent A, wherein 2-phenylpyridine and trichlorohydrin The molar ratio of iridium is 2.3:1, the mixed solvent A is a mixture of 30mL deionized water and 100mL ethylene glycol ether, under the conditions of protective gas nitrogen atmosphere, magnetic stirring (stirring speed 400r / min), and temperature 125°C Reflux for 18 hours, filter to obtain filtrate A and solid A, wash the solid with deionized water, ethanol, and acetone for 4 times, then dissolve in dichloromethane to remove impurities, filter to obtain filtrate B and solid B, and concentrate and dry the filtrate in vacuo The obtained iridium dichloro bridge complex, mass spectrometry (MS / ESI + ) is 1075, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com