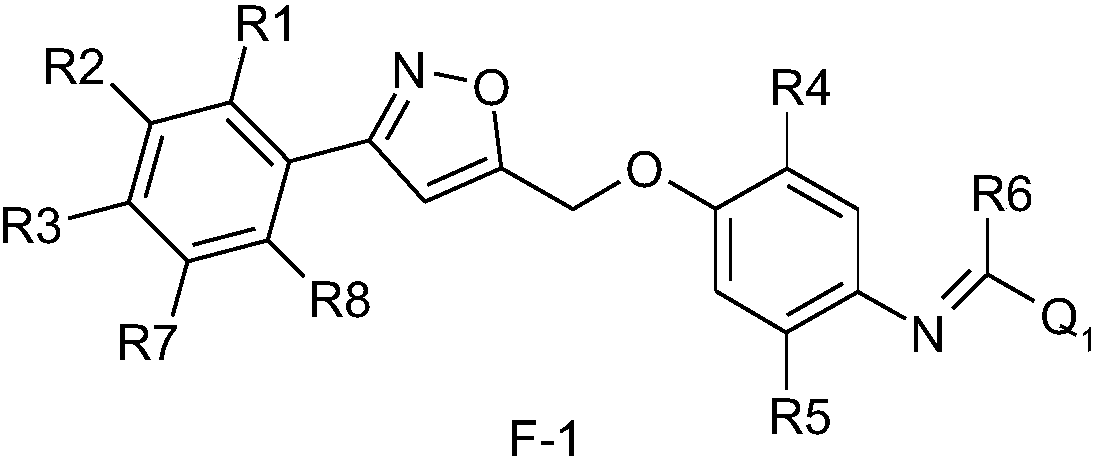
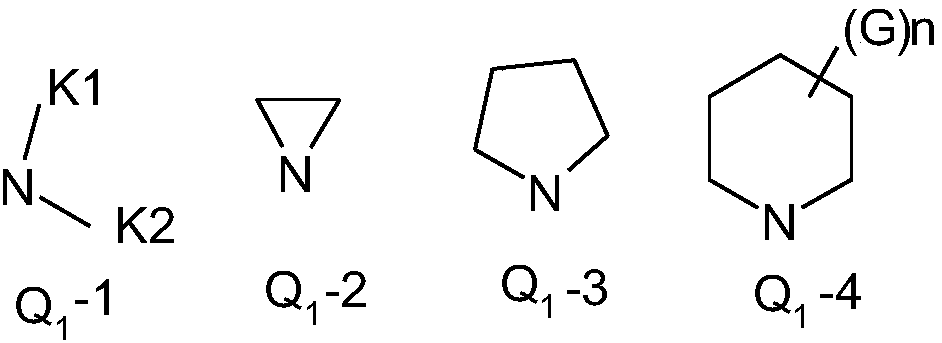
Substituted aromatic diisooxazoline-containing aromatic amidine compounds, and preparation method and application thereof
A technology of isoxazole aryl amidines and compounds, applied in botany equipment and methods, applications, organic chemistry, etc., capable of solving problems such as undisclosed compounds and activities of undisclosed compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1: Synthesis of compound number 2
[0090] The synthetic route is as follows:
[0091]
[0092] (1) Synthesis of intermediate 2e
[0093] Weigh 28.10g (0.20mol) of raw material 1e (o-chlorobenzaldehyde) into a 500mL single-necked flask, add 200mL of absolute ethanol to it, and then dissolve 20.85g (0.30mol) of hydroxylamine hydrochloride in 70mL of water. Sodium carbonate was neutralized and slowly added dropwise to the reaction flask. After the addition was completed, the reaction was refluxed and stirred for 2 hours. TLC followed the reaction process. After the raw materials were completely reacted, the reaction solution was poured into water. A large amount of white solid precipitated out and washed with water. After drying, the product 2e was 29.73g, and the yield was 95.6%.
[0094] The m.p.75~77℃ of product 2e, the nuclear magnetic data is: 1 H NMR(600MHz, CDCl 3 / TMS)δ:7.35~7.43(m,2H,Ar-H), 7.50(d,1H,J=8.0Hz,Ar-H),7.81(d,1H,J=8.0Hz,Ar-H), 8.36 (s, 1H, CH=N), 11...
Embodiment 2、30
[0111] Example 2. 30% suspension concentrate
[0112]
[0113] The compound No. 2 and other components are thoroughly mixed to obtain the suspension. The resulting suspension can be diluted with water to obtain any desired concentration of dilution.
Embodiment 3、30
[0114] Example 3. 30% aqueous suspension
[0115]
[0116] The compound No. 52 is crushed together with 80% of the water to be added and sodium lauryl sulfonate in a ball mill. The semi-fiber and propylene oxide are dissolved in the remaining 20% of water, and then the above components are added with stirring to obtain 30% aqueous suspension.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com