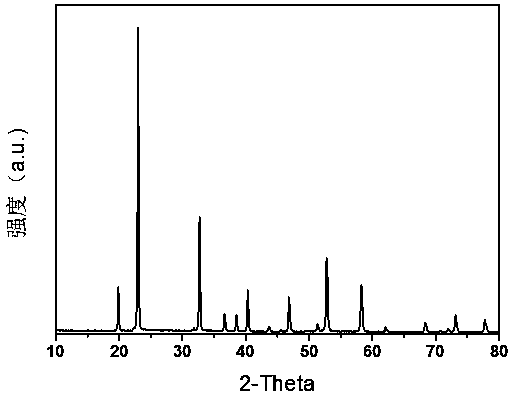
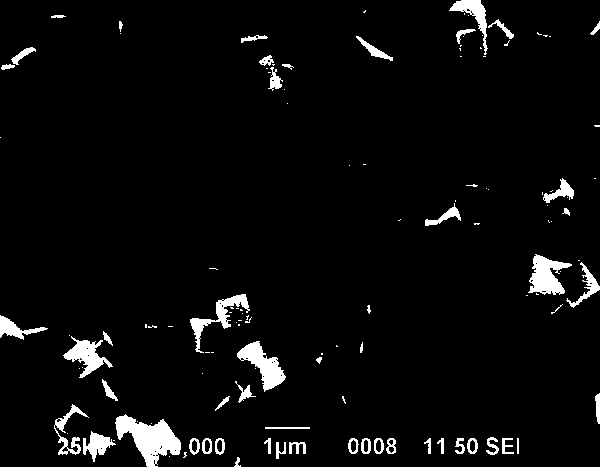
Method for preparing cubic structured zinc stannate photocatalyst responding to sunshine
A technology of structural zinc stannate and photocatalyst, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of low utilization efficiency and limit the industrial application of nano-TiO2 and other problems, to achieve the effect of high degradation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] 2.1036g SnCl 4 ·5H 2 O was dissolved in 30mL ethanol to obtain solution A; 0.8178g ZnCl 2 Dissolve in 60mL water to obtain solution B; mix solution A and solution B, adjust the pH of the mixed system to 3 with 2mol / L sodium hydroxide solution, then filter the mixed solution, wash with water, and dry to obtain zinc stannate photocatalyst , the zinc stannate photocatalyst was exposed to natural sunlight for 5 hours, and the removal rate of methylene blue was 11.05%.
Embodiment 2
[0014] 2.1036g SnCl 4 ·5H 2 O was dissolved in 30mL ethanol to obtain solution A; 0.8178g ZnCl 2 Dissolve in 60mL water to obtain solution B; mix solution A and solution B, adjust the pH of the mixed system to 7 with 2mol / L sodium hydroxide solution, then filter the mixed solution, wash with water, and dry to obtain zinc stannate photocatalyst , the zinc stannate photocatalyst was exposed to natural sunlight for 5 hours, and the removal rate of methylene blue was 11.05%.
Embodiment 3
[0016] 2.1036g SnCl 4 ·5H 2 O was dissolved in 30mL ethanol to obtain solution A; 0.8178g ZnCl 2 Dissolve in 60mL water to obtain solution B; mix solution A and solution B, adjust the pH of the mixed system to 9 with 2mol / L sodium hydroxide solution, then filter the mixed solution, wash with water, and dry to obtain zinc stannate photocatalyst , the zinc stannate photocatalyst was exposed to natural sunlight for 5 hours, and the removal rate of methylene blue was 42.29%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com