Compound for OLED (Organic Light Emitting Diode) material and preparation method of compound
A technology of compounds and equations, applied in the field of optoelectronic functional materials of this type of compounds, in the field of fluorene-fluorene group compounds OLED organic molecular light-emitting materials, can solve very different problems, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
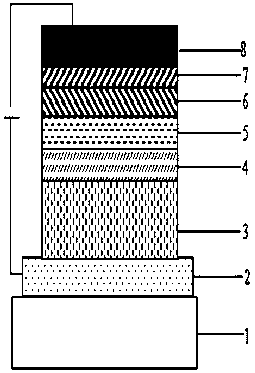
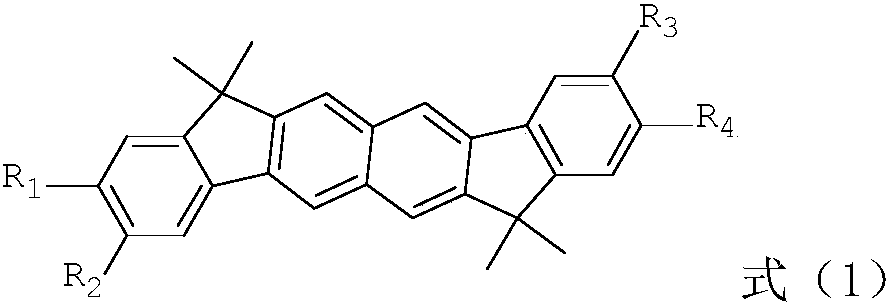
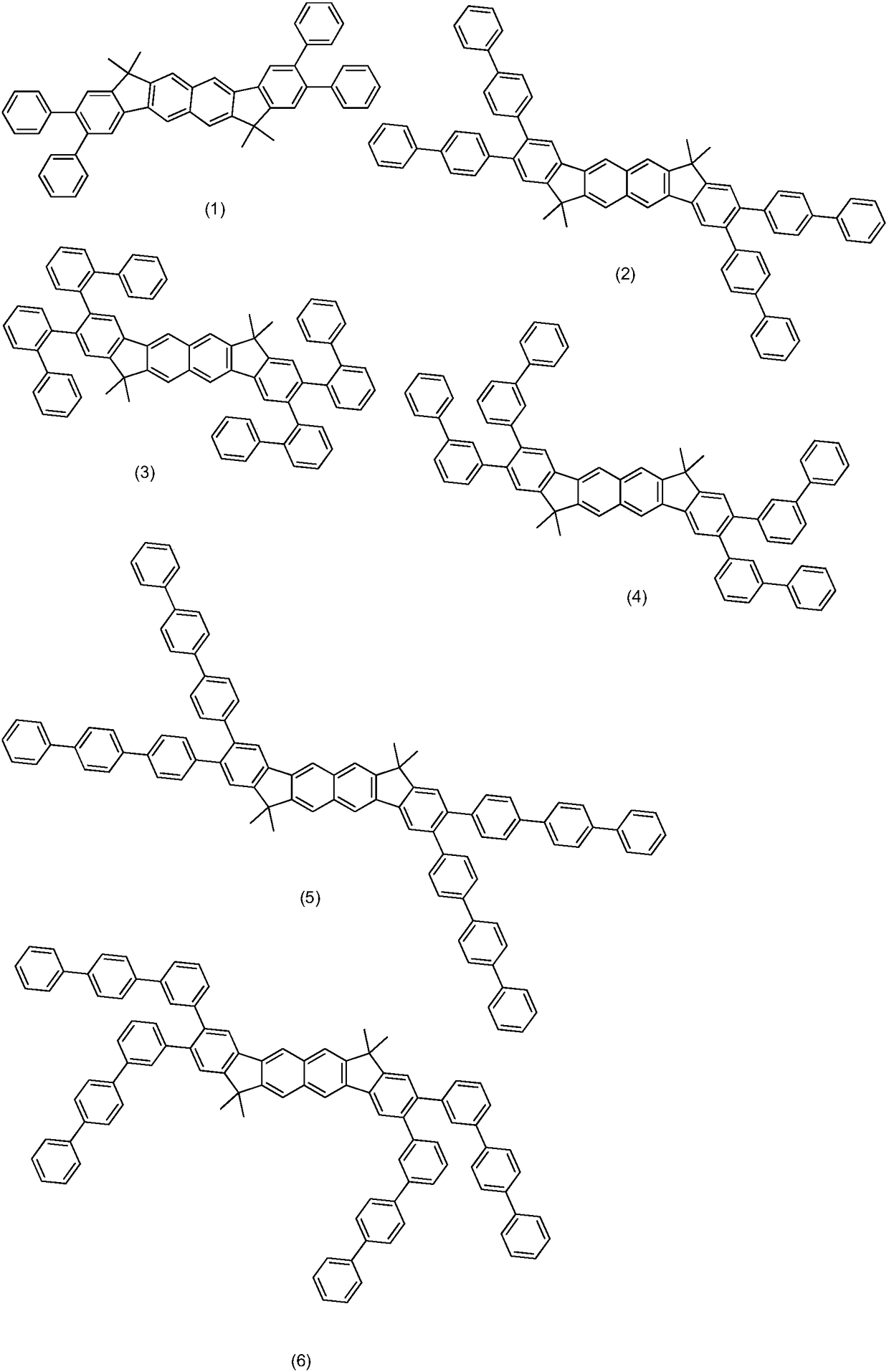
Image
Examples
Embodiment 1
[0063] Embodiment 1: the synthesis of compound 6:
[0064] Synthesis of intermediate compound 3:
[0065]
[0066] Under nitrogen protection, add 21.6g (0.1mol) of compound A, 38.1g (0.1mol) of compound 1, 48.1g (0.1mol) of compound 2, 11.2g (0.2mol) of potassium hydroxide, and 16.8g of water into a 1000ml three-necked flask , Toluene 500g, Pd(PPh 3 ) 4 1.15g (0.001mol), start stirring, heat with an oil bath, control the temperature at 70-80°C and keep it warm for 6-8h. After the reaction is complete after TLC detection, separate layers, wash with water, and remove the solvent to obtain the crude product, add the theoretical amount of the product and equal weight Compound 3 was obtained by recrystallization from toluene and ethanol, and 62.2 g of compound 3 was obtained by drying at 95°C, with a yield of 75.1%.
[0067] Synthesis of intermediate compound 4:
[0068]
[0069] Under the protection of nitrogen, add 62.2g (0.0751mol) of compound 3, 51.6g (0.3mol) of p-to...
Embodiment 2
[0076] Embodiment 2: the synthesis of compound 12:
[0077] Synthesis of intermediate compound 9:
[0078]
[0079] Under nitrogen protection, add 21.6g (0.1mol) of compound A, 750.3g (0.0011mol) of compound 8, 58.3g (0.1mol) of compound 8, 27.6g (0.2mol) of potassium carbonate, 55.2g of water, and toluene into a 1000ml three-necked flask. 500g, Pd(PPh 3 ) 4 2.3g (0.002mol), start stirring, heat with an oil bath, control the temperature at 70-80°C and keep it warm for 6-8h. After the reaction is complete after TLC detection, separate layers, wash with water, and remove the solvent to obtain the crude product, add the theoretical amount of the product and equal weight Compound 9 was obtained by recrystallization from toluene and ethanol, and 75.6 g of compound 9 was obtained by drying at 95°C, with a yield of 75.0%.
[0080] Synthesis of intermediate compound 10:
[0081]
[0082] Under the protection of nitrogen, add 75.6g (0.075mol) of compound 9, 51.6g (0.3mol) of...
Embodiment 3
[0089] Embodiment 3: the synthesis of compound 18:
[0090] Synthesis of intermediate compound 15:
[0091]
[0092] Under nitrogen protection, add compound A 21.6g (0.1mol), compound 13 64g (0.12mol), compound 14 58.1g (0.1mol), potassium carbonate 27.6g (0.2mol), water 55.2g, toluene 600g, Pd(PPh 3 ) 4 1.15g (0.001mol), start stirring, heat with an oil bath, control the temperature at 70-80°C and keep it warm for 6-9h. After the reaction is complete after TLC detection, separate layers, wash with water, and remove the solvent to obtain the crude product, add the theoretical amount of the product and equal weight Compound 15 was obtained by recrystallization from toluene and ethanol, and 86.5 g of compound 15 was obtained by drying at 95°C, with a yield of 80%.
[0093] Synthesis of intermediate compound 16:
[0094]
[0095] Under the protection of nitrogen, add 86.5g (0.08mol) of compound 15, 28.8g (0.3mol) of methanesulfonic acid, and 1000g of toluene to a 2000ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com