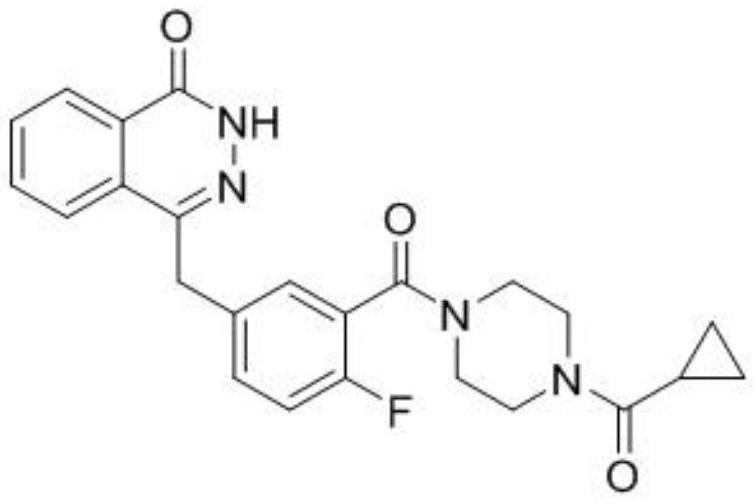
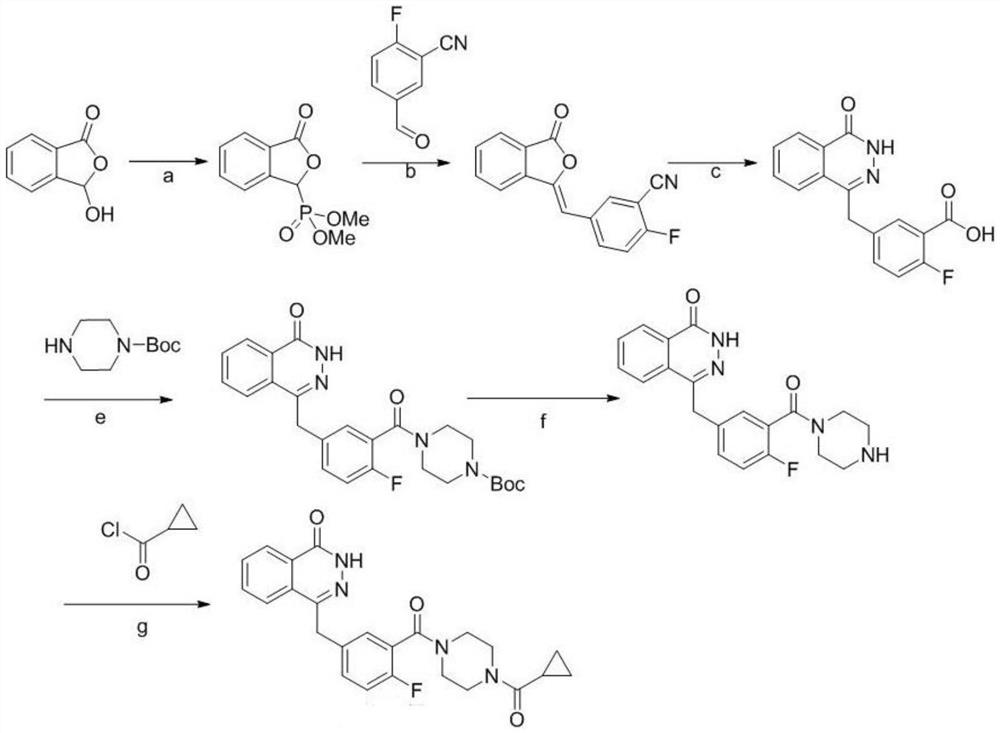
A kind of synthetic method of olaparib
A synthesis method and carbonyl technology, applied in the field of pharmaceutical preparation, can solve the problems of high industrial cost, many steps and high production cost, and achieve the effects of reducing production cost, mild reaction conditions and short reaction route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
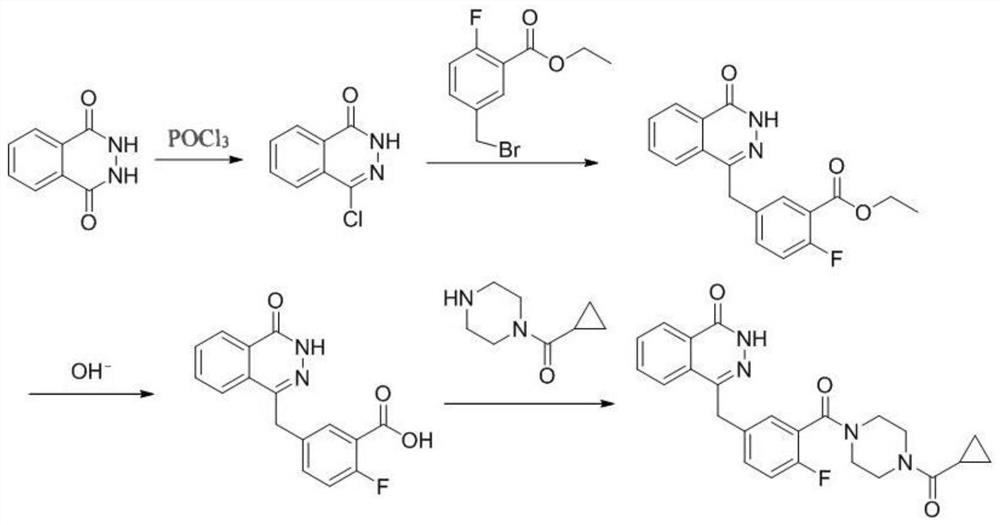
[0045] A synthetic method of olaparib, comprising the following steps:
[0046] (1) Weigh phthalic hydrazide (8.1g, 0.05mol) and add POCl 3 (50mL, 0.25mol), react at 110°C for 4h, after the reaction system is cooled, pour the reaction system into ice water, a white solid precipitates, stir overnight, filter with suction, dry the filter cake, wash with ethyl acetate and filter cake, and finally obtained 8.5 g of 1-chloro-4-carbonylphthalazine with a yield of 94.4%.
[0047](2) Weigh zinc powder (654mg, 0.01mol), add solvent anhydrous tetrahydrofuran 3mL, add 50μL of 1,2-dibromoethane, stir at 60°C for 15 minutes, cool to room temperature, add 50μL of trimethyl At 0°C, 2-fluoro-5-bromomethylbenzoic acid ethyl ester (1.305g, 5mmol) was added dropwise to 3mL tetrahydrofuran solution, and the reaction was continued at 0°C for 2h to obtain a zinc reagent solution. Weigh the product (0.38 g, 2 mmol) in step (1), add 3 mL of tetrahydrofuran, 38 mg of tetrakis(triphenylphosphine)pall...
Embodiment 2
[0051] The molar ratio of phthalic hydrazide and phosphorus oxychloride in step (1) is 1:4 (i.e. phthalic hydrazide 8.1g, phosphorus oxychloride 40mL), other steps are the same as embodiment 1, obtain 8.4 g of 1-chloro-4-carbonylphthalazine, the yield was 93.2%.
Embodiment 3
[0053] The molar ratio of phthalic hydrazide and phosphorus oxychloride in step (1) is 1:3 (i.e. phthalic hydrazide 8.1g, phosphorus oxychloride 30mL), other steps are the same as embodiment 1, obtain 7.9 g of 1-chloro-4-carbonylphthalazine, the yield was 87.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com