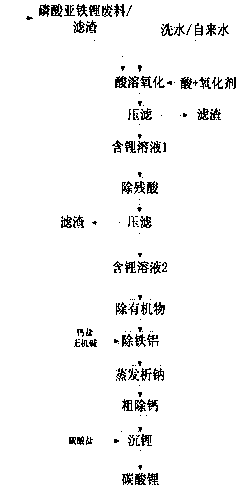
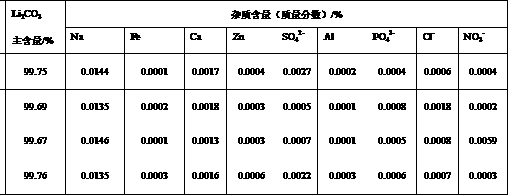
Method for recovering lithium from lithium iron phosphate waste materials for preparing cell-level lithium carbonate
A lithium ferrous phosphate, battery-grade technology, applied in the field of preparation of battery-grade lithium carbonate, can solve the problems of low production efficiency, low product purity, and low lithium yield, and achieve low production cost, high product purity, and lithium recovery high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A. Take 100kg of lithium iron phosphate waste (Li% is 4.1%), add 150L of tap water, and add 39kg of 98% (wt%) H 2 SO 4 , then add 10kg 99% (wt%) sodium chlorate, reaction time 1h, filter to obtain lithium-containing solution 1 with a volume of V 1 =310L;
[0051] Table 1 Chemical composition of lithium-containing solution 1
[0052] element
Li
Al
Fe
Na
Ca
pH
Contentg / L
12.2
4.2
8.9
0.3
0.8
1.8
[0053] B. Add 100 kg of lithium iron phosphate waste to the lithium-containing solution 1 obtained in step A, stir and react for 30 minutes, the end point pH is 4.5, filter to obtain lithium-containing solution 2 and filter residue, and return the filter residue to step A for acid-dissolved oxidation reaction;
[0054] C. Add 1.9 kg of activated carbon to the lithium-containing solution 2 obtained in step B, control the temperature at 20° C., stir for 2 hours, and filter to obtain lithium-containing solution 3; ...
Embodiment 2
[0060] A. Take 100kg of filter residue (Li% is 3.7%), add 350L of washing water, add 84kg of 31% (wt%) HCl, and then add 60kg of 50% (wt%) hydrogen peroxide, the reaction time is 2h, and filter to obtain lithium-containing The volume of solution 1 is V 2 =430L;
[0061] Table 2 Chemical composition of lithium-containing solution 1
[0062] element
Li
Al
Fe
Na
Ca
pH
Contentg / L
8.9
2.8
6.5
0.2
0.5
1.5
[0063] B. Add 100 kg of lithium iron phosphate waste to the lithium-containing solution 1 obtained in step A, stir and react for 90 minutes, the end point pH is 4.0, filter to obtain lithium-containing solution 2 and filter residue, and return the filter residue to step A for acid-dissolved oxidation reaction;
[0064] C. Add 1.8kg of activated carbon to the lithium-containing solution 2 obtained in step B, control the temperature at 100°C, stir for 0.5h, and filter to obtain lithium-containing solution 3;
[0065...
Embodiment 3
[0070] A. Take 100kg of lithium iron phosphate waste (Li% is 3.9%), add 200L of washing water, and add 81kg of 65% (wt%) HNO 3 , then add the sodium hypochlorite of 100kg 10% (wt %), reaction times 1.5h, filter and obtain the volume that contains lithium solution 1 to be V 1 =350L;
[0071] Table 3 Chemical composition of lithium-containing solution 1
[0072] element
Li
Al
Fe
Na
Ca
pH
Contentg / L
11.2
3.8
8.5
0.3
0.6
1.8
[0073] B. Add 100 kg of lithium iron phosphate waste to the lithium-containing solution 1 obtained in step A, stir and react for 60 minutes, the end point pH is 3.0, filter to obtain lithium-containing solution 2 and filter residue, and return the filter residue to step A for acid-dissolved oxidation reaction;
[0074] C. Add 2.1 kg of activated carbon to the lithium-containing solution 2 obtained in step B, control the temperature at 80° C., stir for 1 hour, and filter to obtain lithium-conta...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap