Anti-cancer composition with synergistic effect
A technology of synergistic effect and composition, which can be used in medical preparations containing active ingredients, drug combinations, anti-tumor drugs, etc. The effect of reducing the risk of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
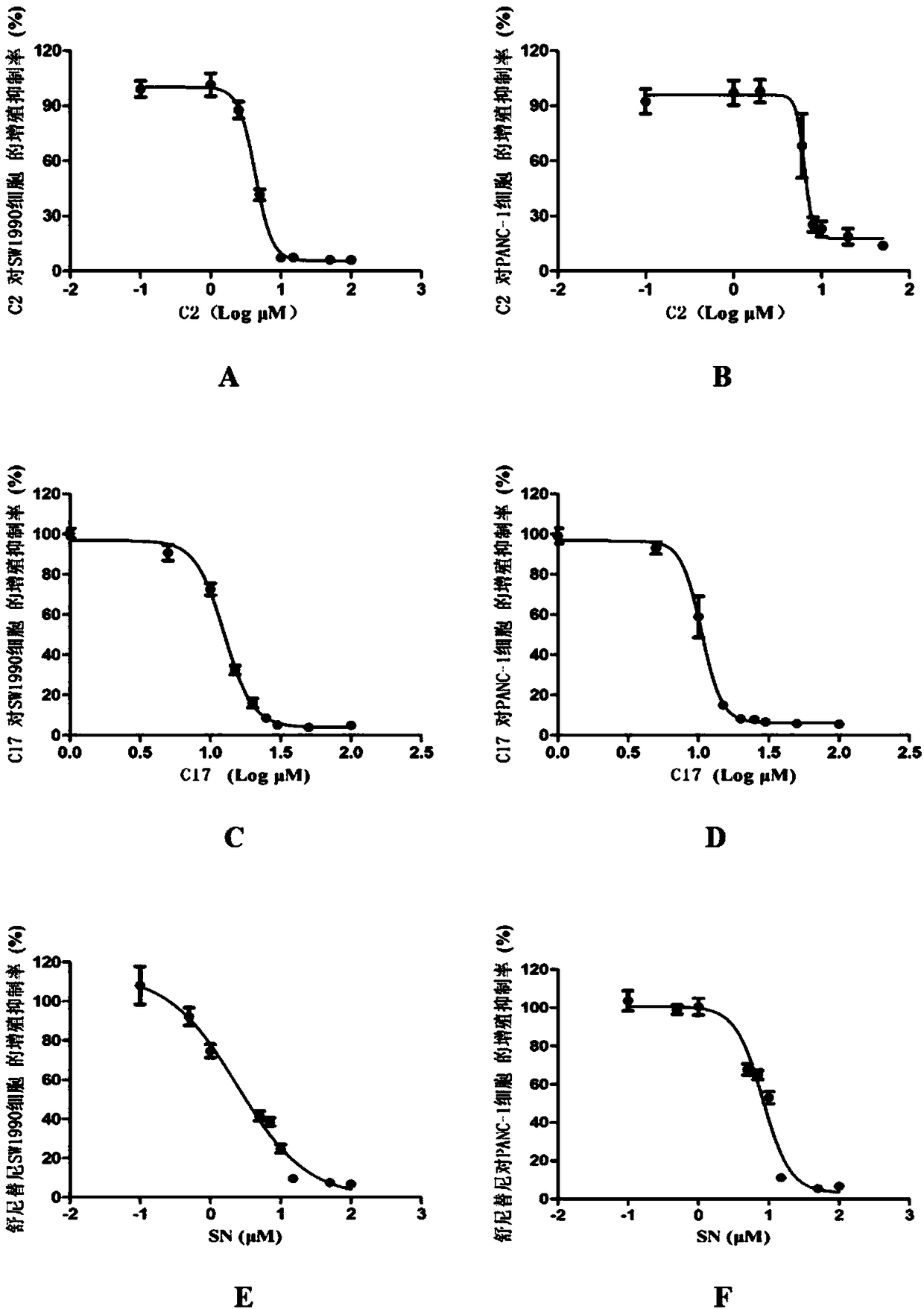
[0030] C2, C17 and SN alone inhibit the proliferation of pancreatic cancer cells
[0031] Take logarithmic growth phase cells, digest with 0.25% trypsin-0.53mmol / L EDTA solution, centrifuge, resuspend and count. Both SW1990 cells and PANC-1 cells were seeded on 96-well cell culture plates at 6000 cells / well. After culturing for 24 hours, the drug was administered. For SW1990 cells, the final concentration of C2 single-drug administration group is: 0.1, 1, 2.5, 5, 10, 15, 50, 100μM; for PANC-1 cells, the final concentration of C2 single-drug administration group is: 0.1, 1, 2, 6, 8, 10, 20, 50μM. In the two cell lines, the final concentration of C17 single-drug administration group was: 1, 5, 10, 15, 20, 25, 30, 50, 100 μM. In the two cell lines, the final concentration of the SN single-drug administration group was: 0.1, 0.5, 1, 5, 7, 10, 15, 50, 100 μM. Each group has 6 parallel holes. Continue to incubate for 48h. After the incubation, the medium was discarded, and 100 μ...
Embodiment 2
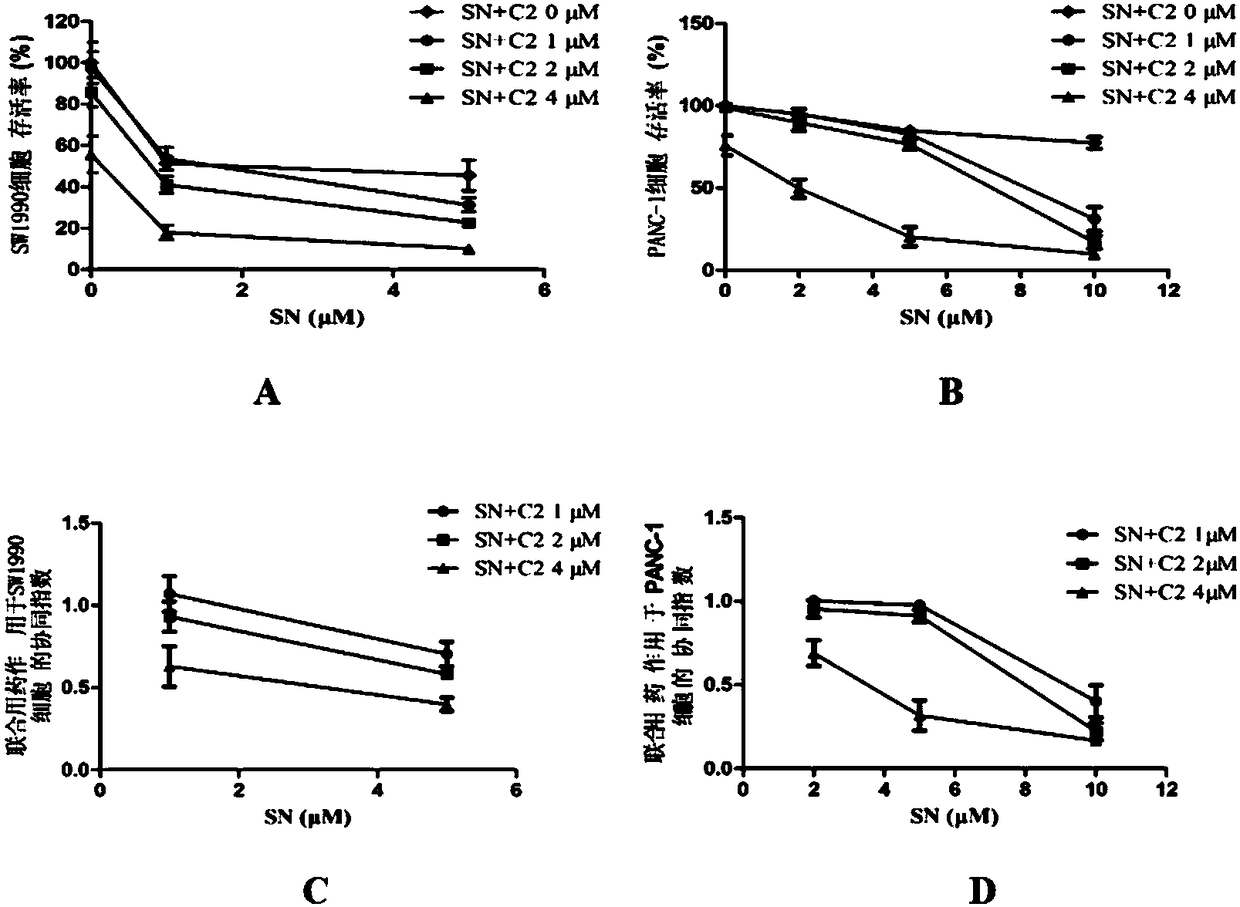
[0035] Inhibitory effect of C2 combined with SN on the proliferation of human pancreatic cancer cells
[0036] Take logarithmic growth phase cells, digest with 0.25% trypsin-0.53mmol / L EDTA solution, centrifuge, resuspend and count. Both SW1990 cells and PANC-1 cells were seeded on 96-well cell culture plates at 6000 cells / well. After culturing for 24 hours, the drug was administered. For the three parallel 96-well plates of SW990 cells, the concentrations of SN are 0, 1, and 5 μmol / L. For the four parallel 96-well plates of PANC-1 cells, the concentrations of SN are 0, 2, 5, 10μmol / L, and in each 96-well plate, the concentration of C2 is set to 0, 1, 2, 4μmol / L, and each group has 6 parallel wells. Continue to incubate for 48h. The absorbance of each well at 540 nm was measured by the SRB method. The method reported in the literature was used to calculate the size of the CI (combination index, CI). Calculate the theoretical absorbance of the combined administration group a...
Embodiment 3
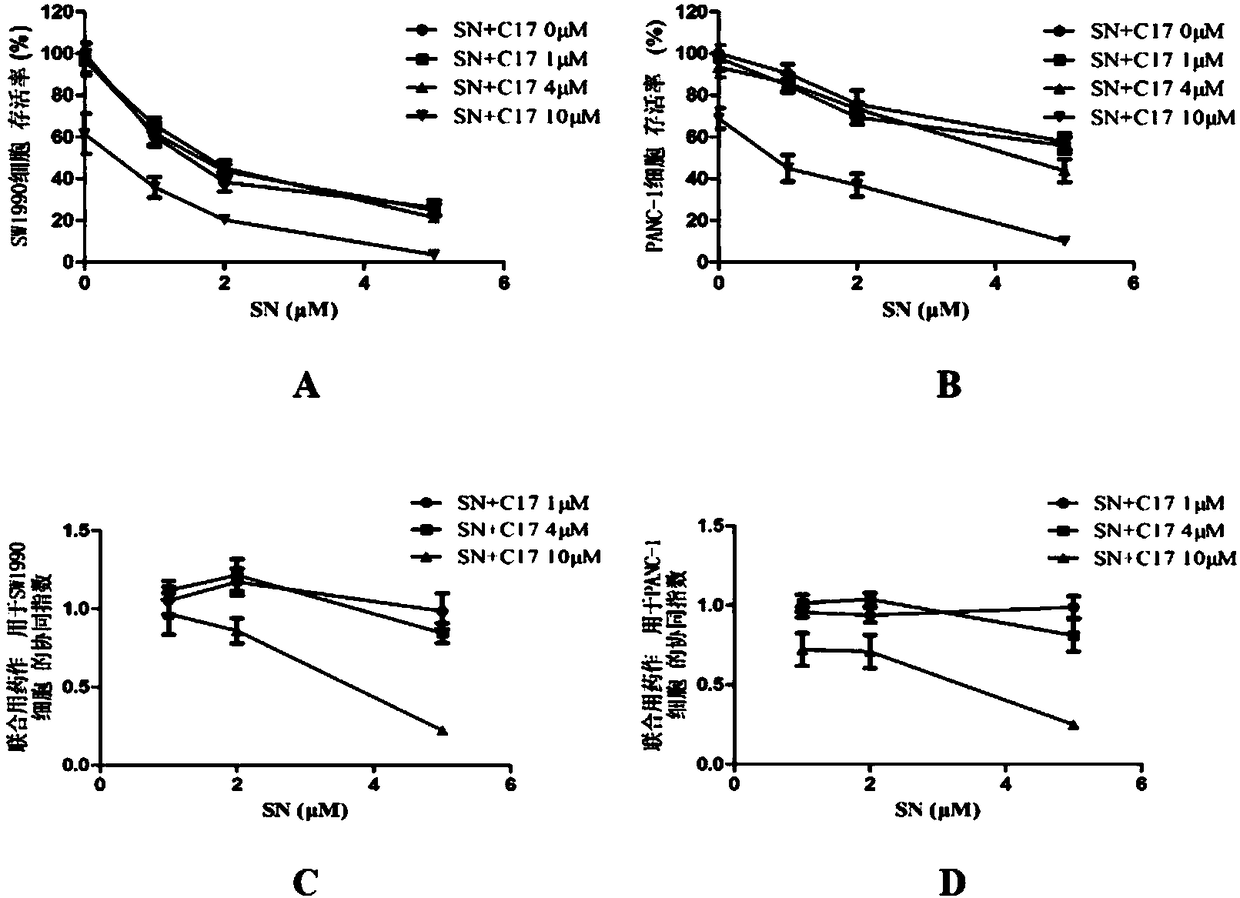
[0041] Inhibitory effect of C17 combined with SN on the proliferation of human pancreatic cancer cells
[0042] Take logarithmic growth phase cells, digest with 0.25% trypsin-0.53mmol / L EDTA solution, centrifuge, resuspend and count. Both SW1990 cells and PANC-1 cells were seeded on 96-well cell culture plates at 6000 cells / well. After culturing for 24 hours, the drug was administered. For the four parallel 96-well plates of two kinds of cells, the concentration of C17 is 0, 1, 4, and 10 μmol / L. In each 96-well plate, the concentration of SN is set to 0, 1, 2, and 5 μmol / L. L, each group has 6 parallel holes. Continue to incubate for 48h. The absorbance of each well at 540 nm was measured by the SRB method. The method reported in the literature was used to calculate the size of the CI (combination index, CI). Calculate the theoretical absorbance of the combined administration group according to formula 3.1, and the ratio of the measured absorbance to the theoretical value i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com