Preparation method and application of melphalan dimmer hydrochloride
A technology of dimer and melphalan, which is applied in the field of preparation and application of melphalan dimer hydrochloride, can solve problems such as unretrieved, and achieve improved stability, mild and controllable preparation conditions, and reaction conditions Gentle and Controllable Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
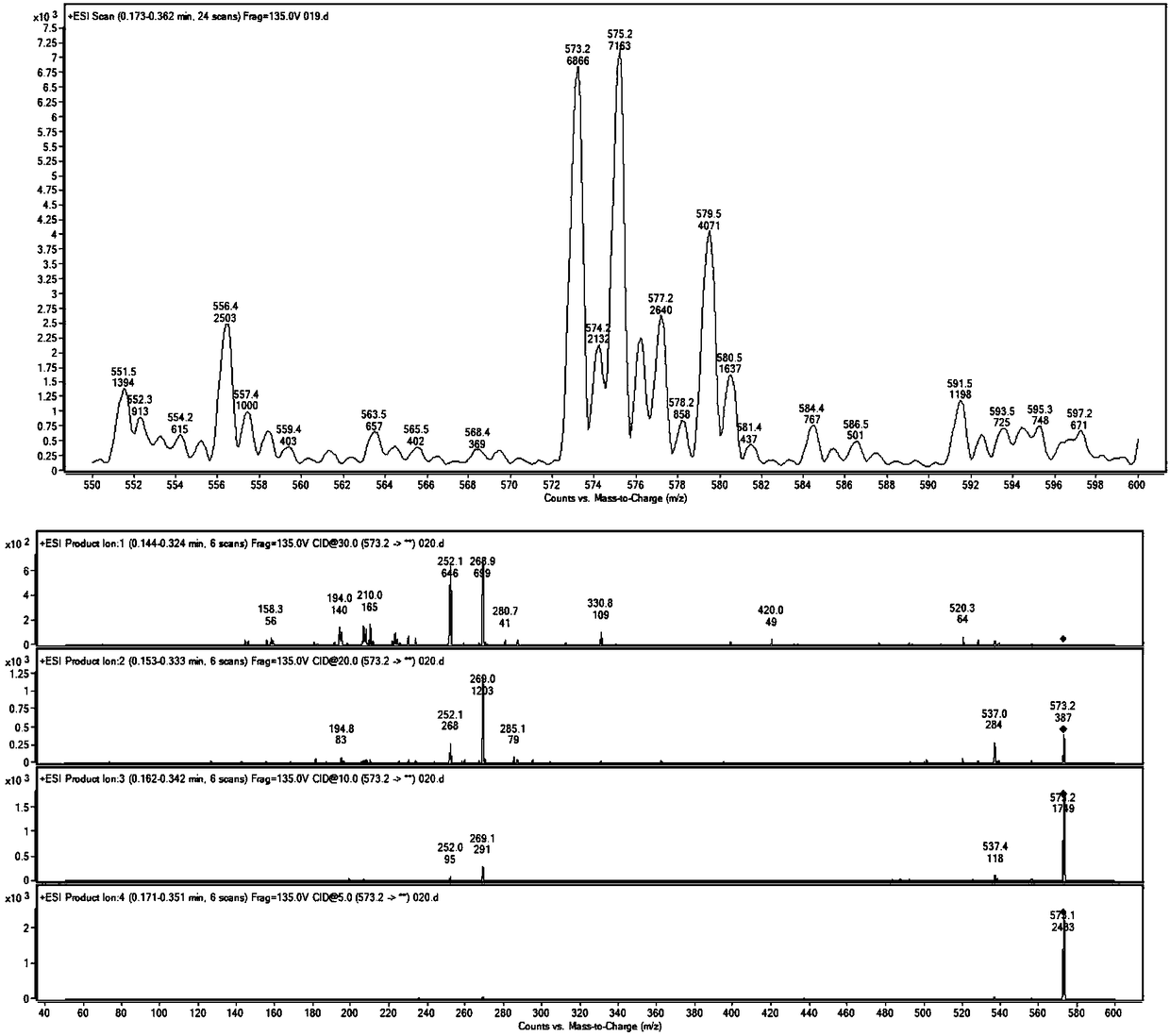
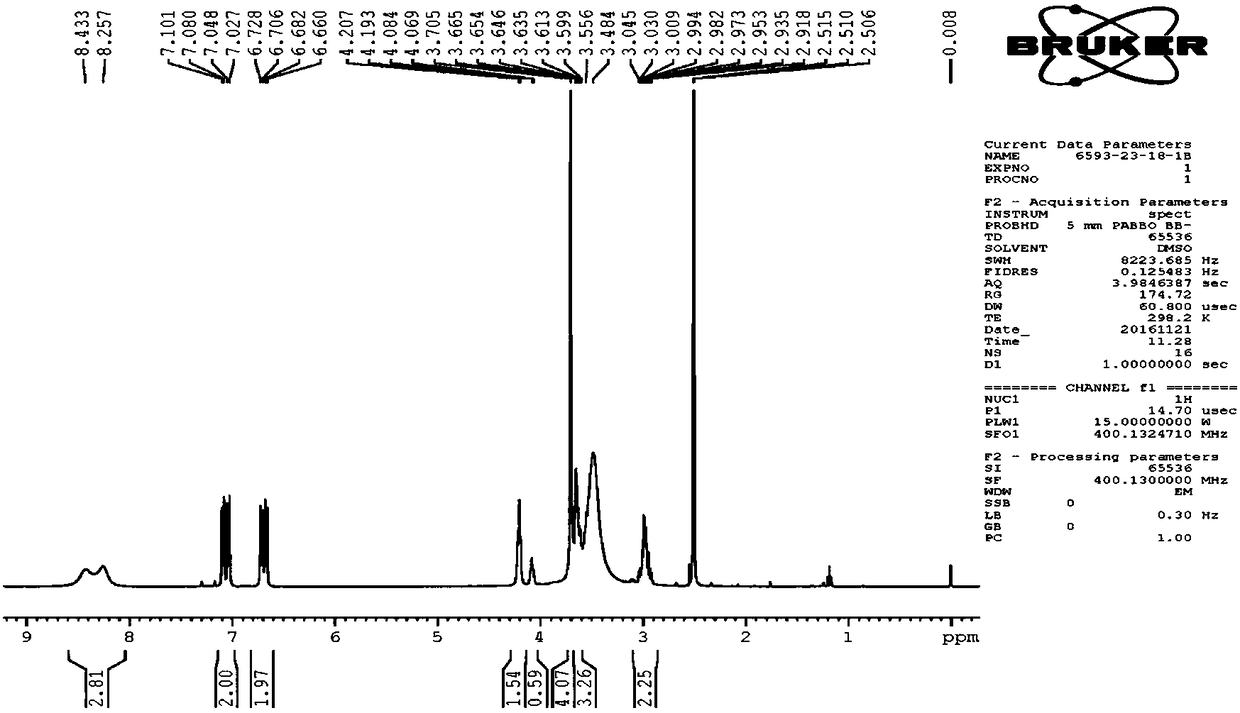
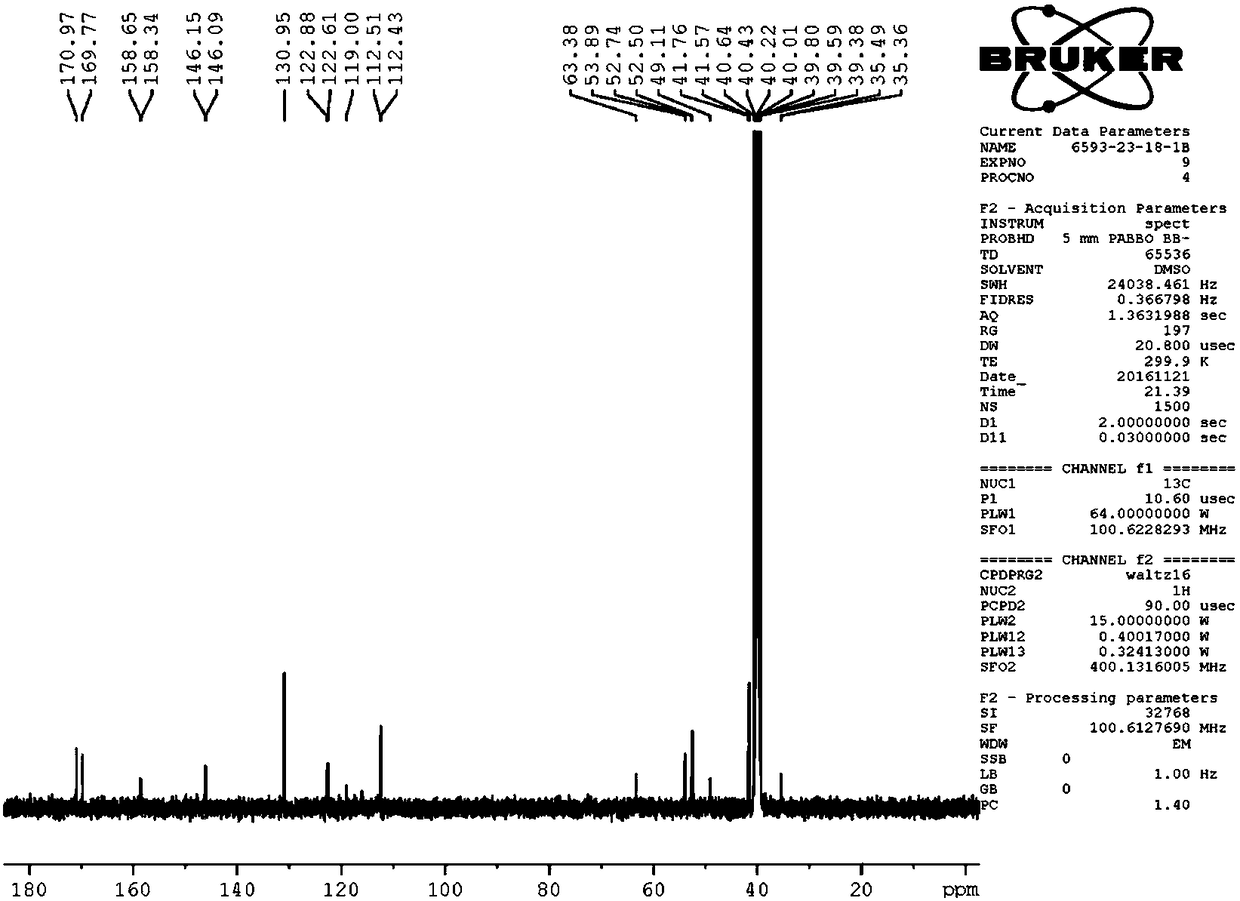
Image
Examples
Embodiment 1
[0038] Add the compound of formula 1 (1g) into the reaction bottle, then add 10ml of dichloromethane, 0.2ml of N,N-dimethylformamide, stir, cool down to 5°C, add thionyl chloride dropwise, react for 1 hour, concentrate The solvent was removed to obtain 0.82 g of compound 2.
Embodiment 2
[0040] Add the compound of formula 1 (1g) into the reaction flask, then add 12ml of dichloromethane, 0.2ml of N,N-dimethylformamide, stir, raise the temperature to 40°C, add dropwise oxalyl chloride, react for 2 hours, concentrate to remove the solvent , 0.68 g of compound 2 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com