Nitroxyl radical covalent organic framework material as well as synthetic method and application thereof
A technology of covalent organic framework and nitroxide radicals, applied in the field of nitroxide radical covalent organic framework materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
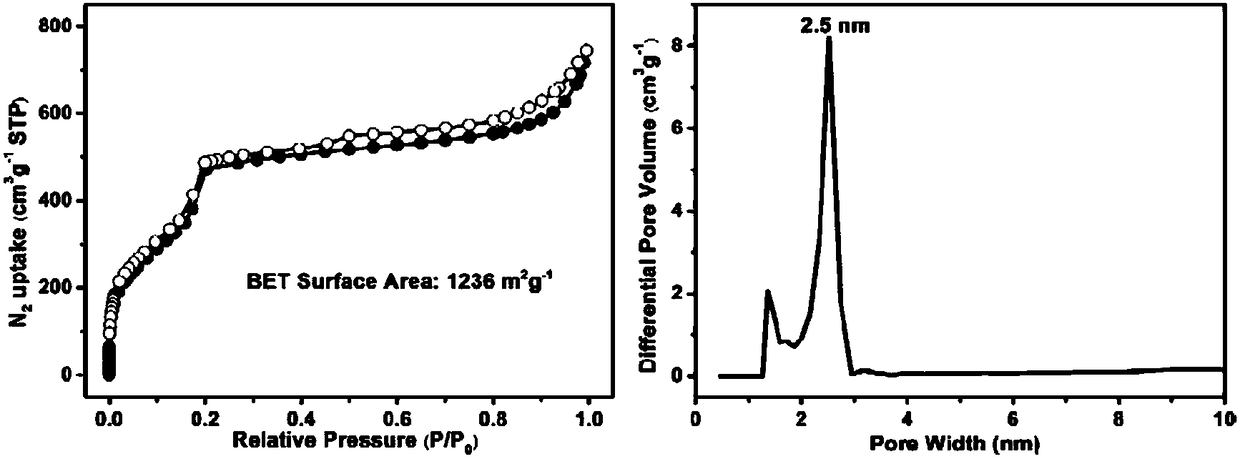
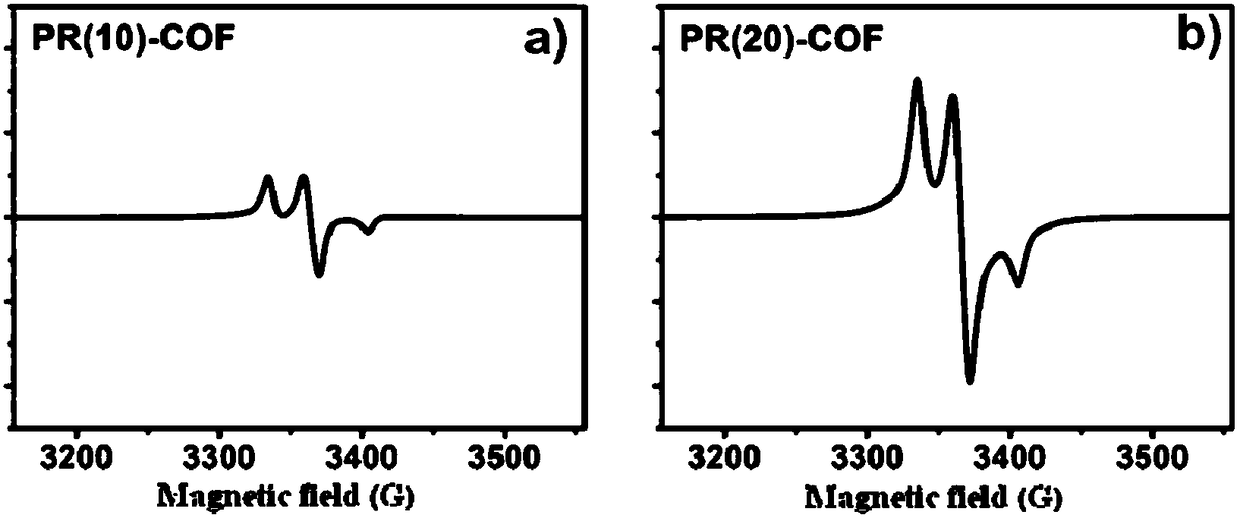
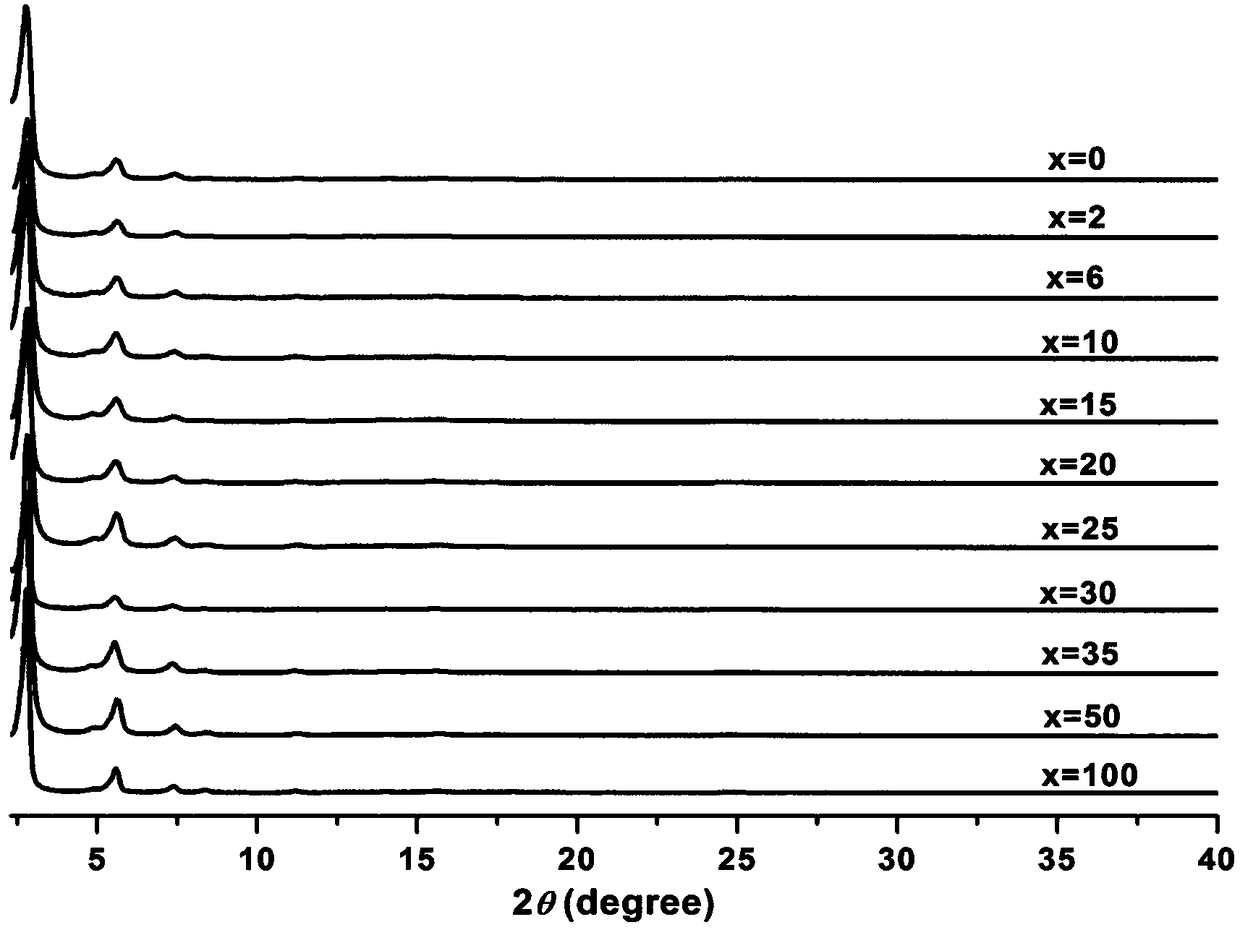
[0030] 15.3 mg of trimesaldehyde and 4.5 mg of radical precursor 1 and 38.6 mg of non-radical precursor 2 were added to the pressure-resistant tube. Then add 1mL dioxane, 1mL mesitylene and 1mL ethanol, shake well and add 0.3mL 9mol / L acetic acid solution. After sealing the pressure tube with a rubber stopper, replace it with argon three times, then quickly remove the rubber stopper, and seal the pressure tube with a polytetrafluoroethylene stopper. It was placed in an oven and reacted at 80° C. for five days. After the reaction, a solid was produced at the bottom of the pressure-resistant tube, and the solid was transferred to a centrifuge tube, washed with acetone and tetrahydrofuran for 3 times, respectively. The solid was dried at 80°C to obtain 35.2 mg of brown yellow solid powder PR(10)-COF, with a yield of 68%.
Embodiment 2
[0032] Using 0 mg of free radical precursor 1 and 42.9 mg of non-radical precursor 2, the others were the same as in Example 1 to obtain 35.7 mg of PR(0)-COF with a yield of 69%.
Embodiment 3
[0034] Using 0.9 mg of free radical precursor 1 and 42 mg of non-radical precursor 2, the others were the same as in Example 1 to obtain 33.5 mg of PR(2)-COF with a yield of 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com