Knee joint orthotics capable of preventing lateral twisting force
A twisting force, knee joint technology, applied in the direction of drug devices, devices introduced into the body, hypodermic injection devices, etc., can solve the problems of aggravating knee joint injury, poor user experience, etc. The effect of preventing excessive distortion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of PEGylated Oleanolic Acid / Magnolol Sustained Release Agent
[0041] The PEGylated oleanolic acid was dissolved in a mixed solution of propylene glycol: water = 1:3 to prepare a 5 mg / mL solution. Magnolol was dissolved in a mixed solution of propylene glycol: water = 1:3 to prepare a 5 mg / mL solution. The substrate was soaked in the above magnolol solution for 15 minutes, washed with water for 5 minutes, then soaked in PEGylated oleanolic acid solution for 15 minutes, and washed with water for 5 minutes. This alternate deposition process was repeated to obtain a PEGylated oleanolic acid / magnolol film.
Embodiment 2
[0042] Embodiment 2: Research on release kinetics in vitro
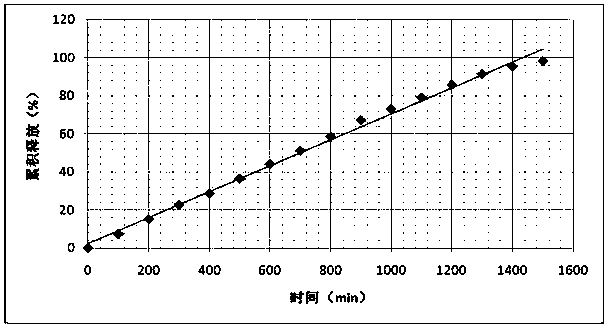
[0043] The PEGylated oleanolic acid / magnolol film obtained in Example 1 was soaked in a phosphate buffer solution for in vitro release experiments. Use 900mL of pH 5.8 phosphate buffer solution as the release medium, the rotation speed is 50 rpm, the temperature of the water bath is 37°C, sample 10mL every 100min, and at the same time supplement the release medium at the same temperature and volume, take out the sample release solution with a 0.45μm micrometer Pore membrane filtration, take the subsequent filtrate as the test sample solution. Detect the concentration of PEGylated oleanolic acid in phosphoric acid buffer solution with high performance liquid chromatography, obtain the release kinetics curve of PEGylated oleanolic acid as follows figure 1 shown. It can be seen that the PEGylated oleanolic acid is always released at a constant rate, without burst release, and can be completely released.
Embodiment 3
[0044] Embodiment 3: the effectiveness experiment of pharmaceutical composition
[0045] 50 cases were selected, aged 40-70 years, 23 males and 27 females, 25 people in each group. Both groups of patients had similar degree of muscle soft tissue swelling and joint pain.
[0046] Treatment group: the drug slow-release preparation of this embodiment was applied to the affected area once a day for 7 consecutive days, and the effect on the 7th day and before treatment was observed.
[0047] Control group: Apply commercially available common joint pain relief ointment to the affected area, once a day, for 7 consecutive days, and observe the effect on the 7th day and before treatment.
[0048] Evaluation method: It is divided into three grades: cured, markedly effective, and ineffective. Cure: the swelling subsides completely, and the pain disappears; markedly effective: the swelling subsides obviously, and the pain relieves; ineffective: the swelling subsides not obviously, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com