Composition of cannabidiol and hydantoin antiepileptic drug and use thereof
A technology of levulinyl urea and antiepileptic drugs, applied in the field of medicine, can solve the problems of reducing the percentage of tonic-clonic seizures, meaningless epilepsy treatment, and no obvious synergistic effect, so as to improve compliance and eliminate side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
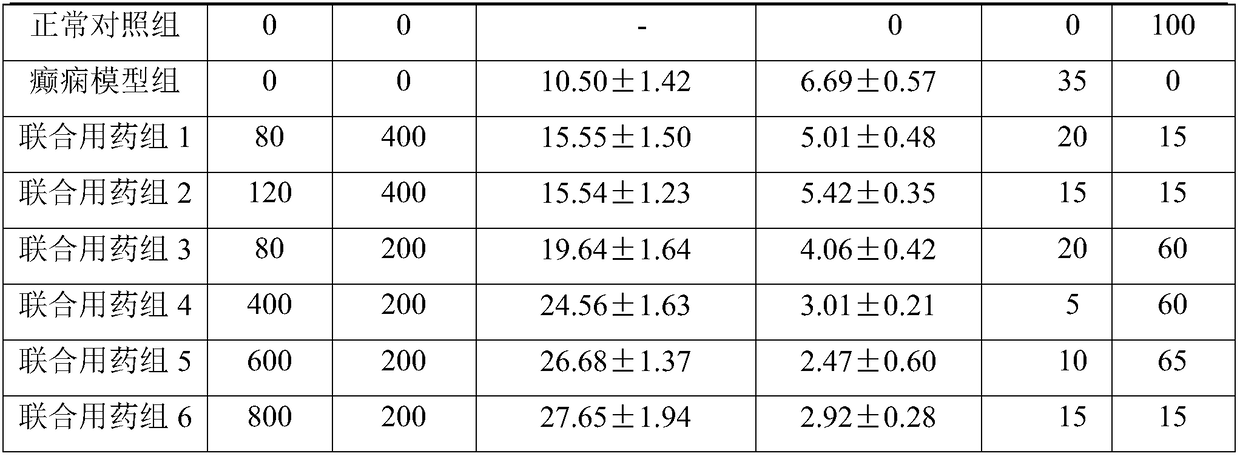
[0042] Example 1 Effect of the combination of CBD and levionylurea antiepileptic drugs on the therapeutic effect of epilepsy
[0043] Experimental Materials
[0044] Experimental animals: healthy male Wistar rats, weighing 75-110 g. Before the experiment, the animals were acclimatized to the experimental environment, cage, injection protocol and handling. The animals were kept at 21°C, 50% humidity and 12 hours of light, with free access to food and water.
[0045] experimental method
[0046] Rat partial seizure model establishment: The rat partial seizure model was induced by penicillin (Bostanci and Bagirici, 2006).
[0047] The experimental animals were divided into random groups, 20 in each group. The vehicle control or the test sample was injected intraperitoneally to the experimental animals. One week prior to this, a cannula was surgically implanted in the right ventricle of the animal under anesthesia. One hour after administration, penicillin (1000 IU / kg) was i...
Embodiment 2
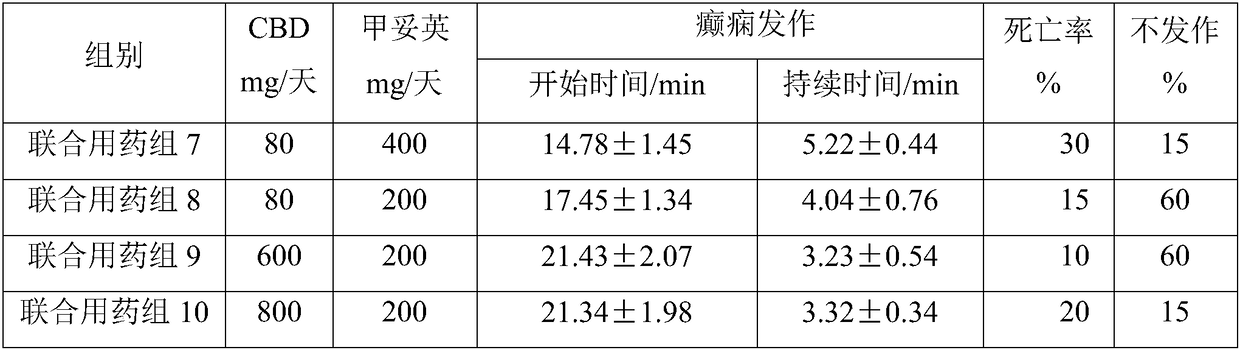
[0066] Example 2 Animal experiments on the effect of the combination of CBD and levionylurea antiepileptic drugs on the side effects of drugs
[0067] Experimental animals: healthy male SD rats, 250-300g. Breeding conditions: room temperature 22±1°C, humidity 50±10%, natural light, free access to food and water. All animals were acclimatized in the feeding environment for 5 days before starting the experiment. Before the experiment, they were fasted for 12-16 hours and had free access to food and water. The experimental animals were randomly divided into 10 groups, 6 animals in each group.
[0068] Normal control group: normal experimental animals, given the same amount of solvent;
[0069] CBD and phenytoin combined administration group 1: animal model of epilepsy ignited by kainic acid, administered with 200mg phenytoin + 60mgCBD / day;
[0070] Combined administration of CBD and phenytoin group 2: animal model of epilepsy ignited by kainic acid, administered with 200 mg ph...
Embodiment 3
[0088] Example 3 Clinical experiments on the effect of the combination of CBD and PHT on the side effects of drugs
[0089] Subjects: 60 volunteers with partial epileptic seizures, male or female, were randomly divided into 2 groups, 20 people in each group, which were the control group and the experimental group.
[0090] Experimental group: 200mgPHT+600mgCBD / day;
[0091] Control group: PHT dosage 200mg / day;
[0092] Experimental method: According to the above-mentioned dosing regimen, the subjects were administered once a day for 12 consecutive months; the frequency of seizures and side effects during the 2nd, 4th, 6th, 8th, 10th, and 12th months were observed and recorded to treat The attack frequency in the first 2 months was used as the basic control.
[0093] Side effects: test blood routine, electrolytes and monitor blood drug concentration, record the side effects that occur at each observation point within 2 months; the side effects include ataxia, drowsiness, head...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com