A kind of synthetic method of highly active macrolide ivorenolide B
A technology of macrocyclic lactone and synthesis method, which is applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems that the cis-trans problem of macrocyclic lactone has not been solved well, the steps are cumbersome, etc., and achieve low synthesis cost, Ease of operation, high yield and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
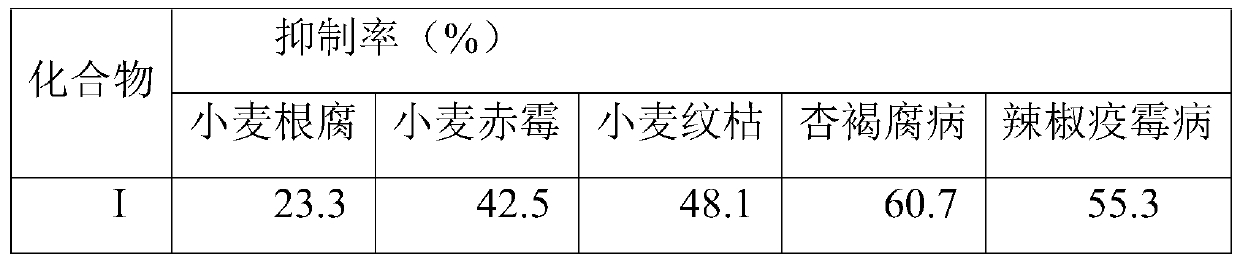
Examples
Embodiment
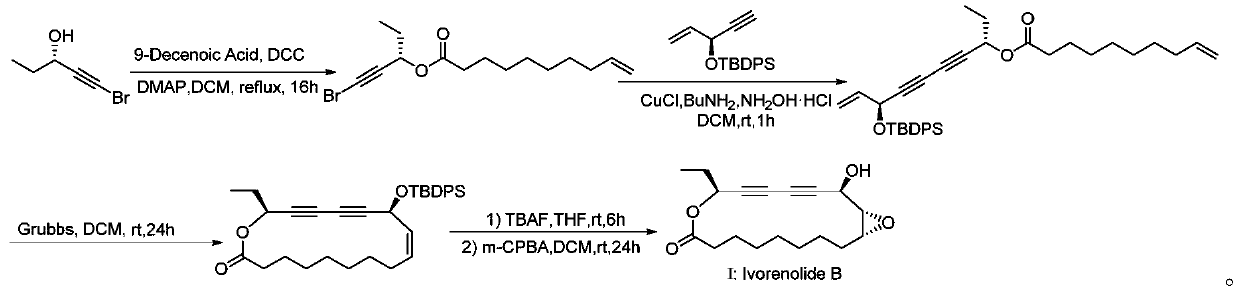
[0028] The synthetic method of the highly active macrolide Ivorenolide B of the present embodiment, the structure of the highly active macrolide Ivorenolide B is The synthetic method of described macrocyclic lactone Ivorenolide B comprises the following steps:
[0029] (1) Synthesis of chiral alcohol esters: In a Shrek tube equipped with a magnetic stirrer, (S)-1-bromo-1-pentyn-3-ol (1.6301g, 10mmol), 9-decane Dienoic acid (1.7025g, 10mmol), dichloromethane 100ml, dicyclohexylcarbodiimide (4.1236g, 20mmol), 4-dimethylaminopyridine (12.3mg, 0.1mmol), after the system was clarified, refluxed for 16h, The progress of the reaction was monitored at any time by thin-layer chromatography. After the reaction was completed, the reaction solution was suction-filtered sequentially, the filter cake was washed with dichloromethane, the combined organic layer was washed with water, washed with sodium chloride, dried over anhydrous sodium sulfate, desolvated and purified by column chromato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com