Radiation-resistant peptide mutant protein and application of mutant protein in reducing inflammatory response
An inflammatory response and protein technology, applied in the fields of peptide/protein components, applications, animal/human proteins, etc., can solve problems such as cold syndrome-like side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
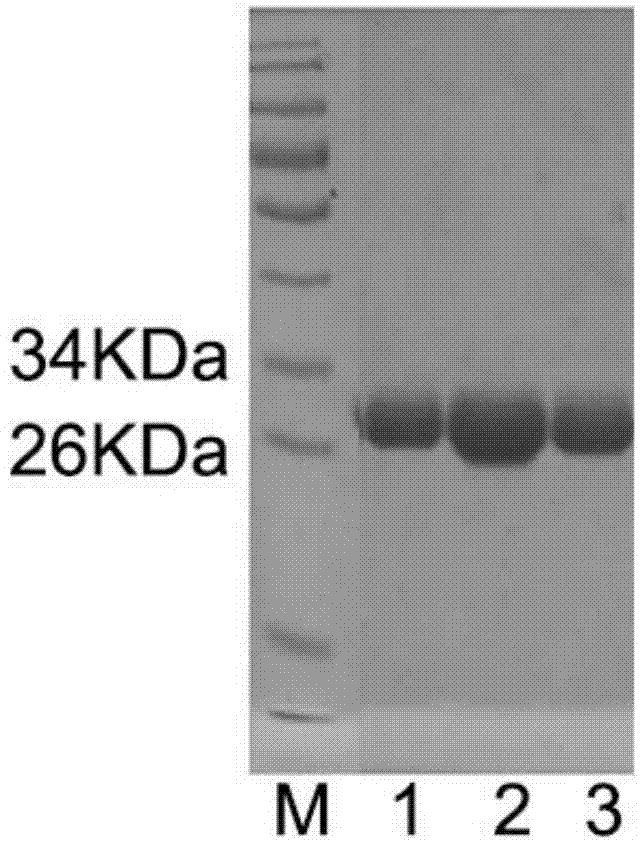
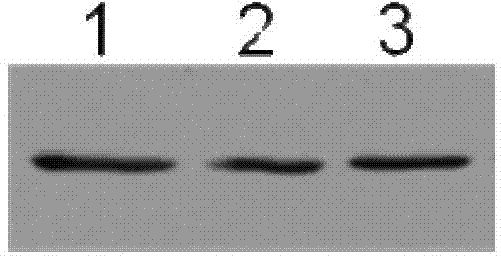
[0037] Example 1, the acquisition of radioresistant peptide mutant L292A
[0038] 1. Acquisition of vector expressing radioresistant peptide mutant L292A
[0039] Synthesize the following point mutation primers:
[0040] Radioantipeptide-I213A-F: TAACCCACTGGCTTCA GC TGATTCTGCATTGTCA
[0041] Radioantipeptide-I213A-R: TGACAATGCAGAATCAGCTGAAGCCAGTGGGTTA
[0042] Radioresistant peptide-L292A-F: TTCCGCAAAACGTC GCG TCTTTACTGCGTTA
[0043] Radioresistant peptide-L292A-R: TAACGCAGTAAAGACGCGACGTTTTGCGGAA
[0044] In order to clone the full-length sequence of the radioresistant peptide into the pBV220 prokaryotic expression vector, the PCR method was used to pMD-18T-FKT (Wang Zhidong et al., CBLB502 protein prokaryotic expression and radiation protection verification, Chinese Journal of Radiation Medicine and Protection, 2010 (30) 4:169-172, which can be obtained from the Institute of Radiation and Radiation Medicine, Academy of Military Medical Sciences of the Chinese People’s...
Embodiment 2
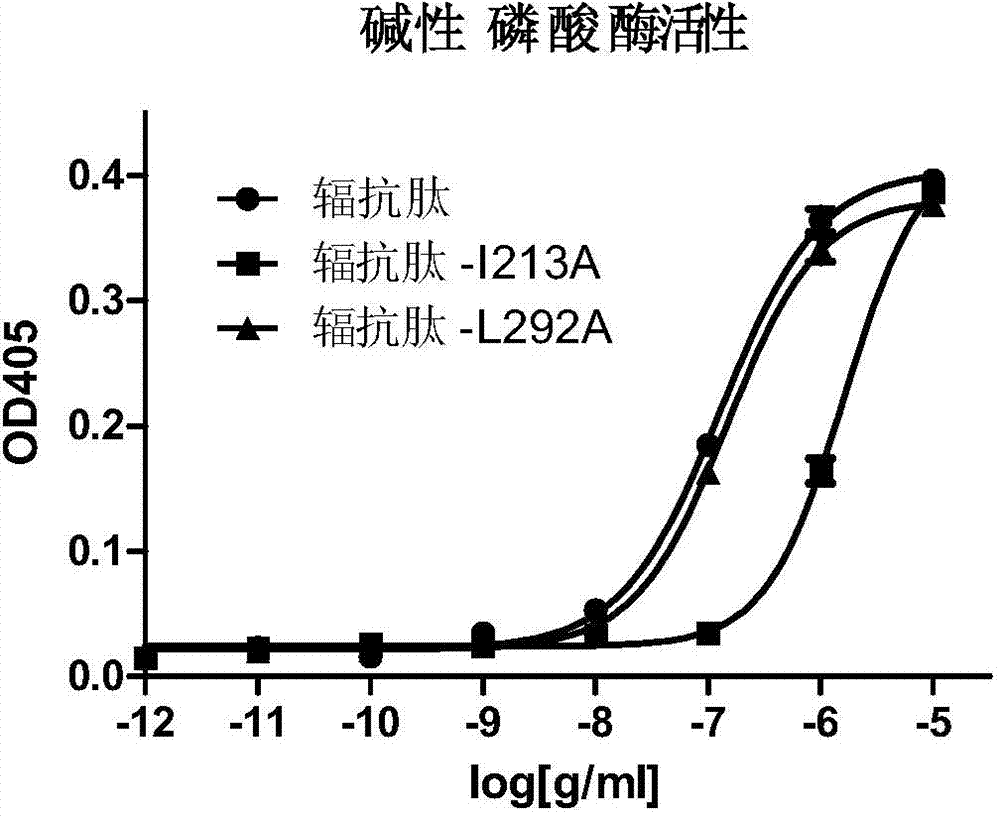
[0060] Example 2. Application of radioresistant peptide mutant L292A in reducing the inflammatory response caused by it
[0061] 1. Activity detection of radioresistant peptide mutant L292A
[0062] Alkaline phosphatase reporter gene detection is used, as follows:
[0063] Resuscitate HEK293T cells and place in an incubator at 37 °C, 5% CO 2 Under normal conditions, cultured in H-DMEM medium. When the 293T cells in the 24-well plate were cultivated until the cell density reached 70%, each well of cells was transfected with 50ng pcDNA-3.1 / hTLR5 plasmid (Wang Lei et al., The radioprotective effect of Toll-like receptor 5 agonist CBLB502 protein, International Journal of Pharmaceutical Research Volume 39, Issue 3, June 2012, 225-230, the public can obtain from the Institute of Radiation and Radiation Medicine, Academy of Military Medical Sciences of the Chinese People's Liberation Army), 100ng100ng pNF-κB / SEAP plasmid (IMGENEX, IMK-515), 2ng pRL -TK plasmid (Promega, E2241) wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com