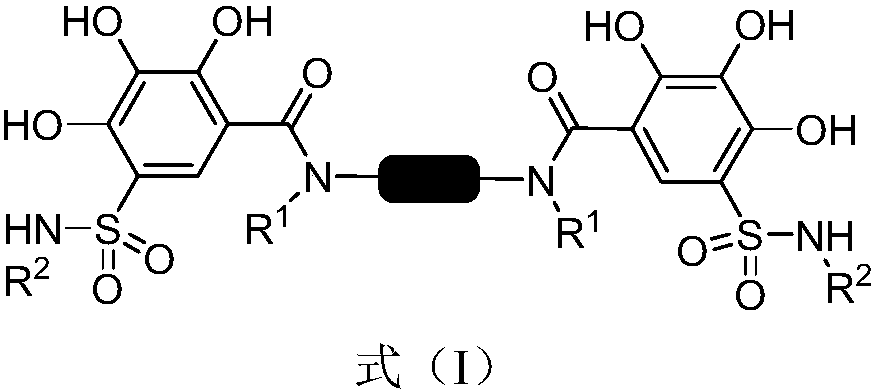
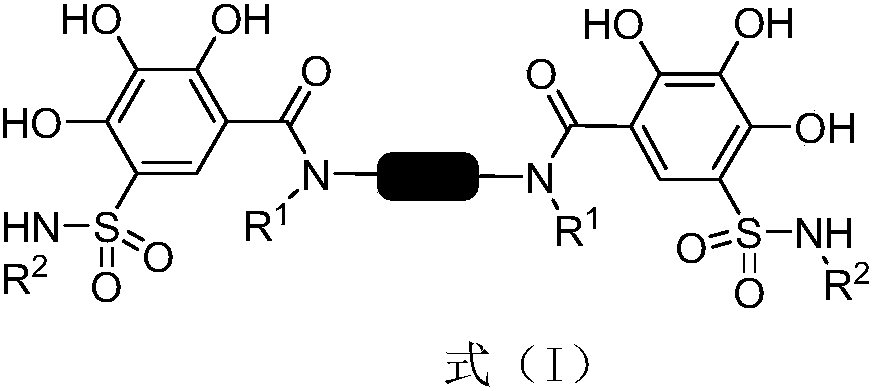
2,3,4-trihydroxybenzenesulfonamide derivatives as well as preparation method and application thereof
A technology for trihydroxybenzenesulfonamide and derivatives, which is applied in the field of 2,3,4-trihydroxybenzenesulfonamide derivatives and their preparation, and can solve the problems of less research and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of N,N'-(1,4-phenylene)bis(2,3,4-trihydroxy-5-(N-propylsulfonamide)benzamide)(lyz003-81)
[0028]
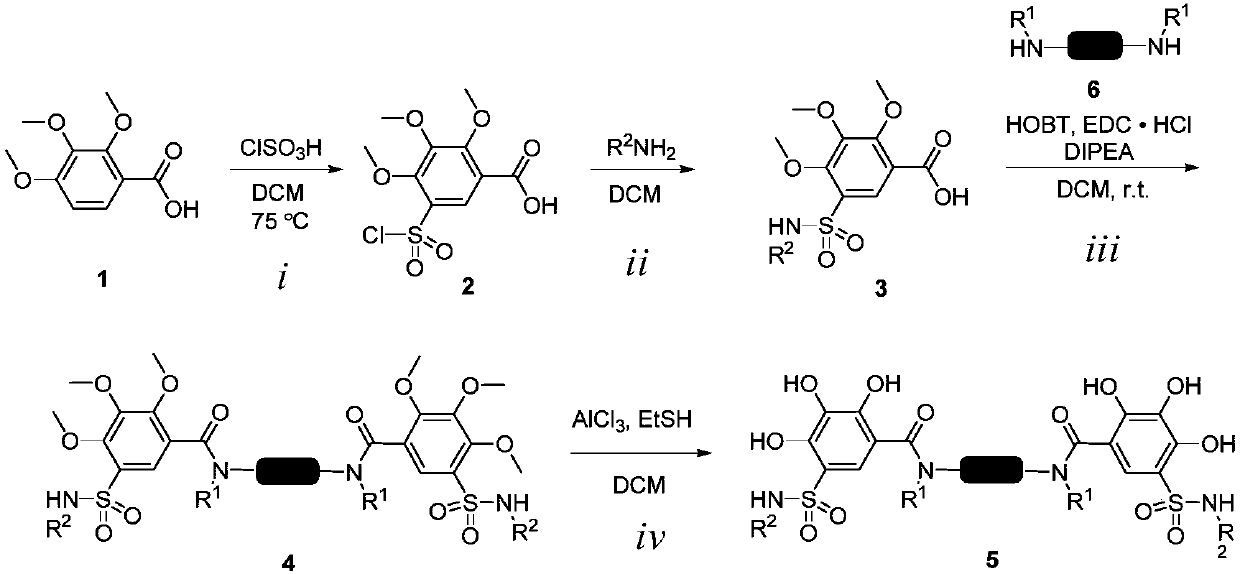
[0029] Under cooling in an ice-water bath, 20 mL of chlorosulfonic acid (3.496 g, 30 mmol, 15.0 eq) in DCM was added dropwise to 5 mL of compound 1 (424 mg, 2 mmol, 1.0 eq) in DCM, after the addition was complete, stir at room temperature for 4 h , and then heated to 75 ° C, reflux 4h. Cool to room temperature, quench with ice water, extract with DCM, and spin dry the obtained reaction product 2. 2mL of n-propylamine (221.7mg, 3.75mmol, 2.5eq) in DCM was added dropwise to 6mL of compound 2 (466mg, 1.5mmol, 1.0eq) in DCM, after the addition was complete, it was stirred at room temperature, and the reaction was detected by TLC. The product was separated by column chromatography as the raw material for the next reaction. The above compound (406.7mg, 1.22mmol, 2.4eq) and EDC (233.9mg, 1.22mmol, 2.4eq) in anhydrous DCM (8mL) were stirred at room temperature fo...
Embodiment 2
[0032] Preparation of N,N'-(1,4-phenylene)bis(2,3,4-trihydroxy-5-(N-isobutylsulfonamide)benzamide)(lyz003-175)
[0033]
[0034] The preparation method was as in Example 1, prepared from compound 2 (800mg, 2.57mmol), isobutylamine (564.6mg, 7.72mmol), p-phenylenediamine (48.7mg, 0.45mmol). Yellow-white solid with a yield of 91%.
[0035] 1 H NMR (400MHz, DMSO-d 6 )δ12.94(brs,1H),10.60(s,1H),8.04(s,1H),7.66(s,2H),6.93(s,1H),2.57–2.54(m,2H),1.69–1.60 (m,1H),0.79(d,J=6.6,6H). 13 CNMR (100MHz, DMSO-d 6 )δ167.69, 153.46, 147.73, 134.06, 133.36, 122.10, 120.01, 118.31, 107.01, 50.09, 27.85, 20.00, 19.89.
Embodiment 3
[0037] Preparation of N,N'-(1,4-phenylene)bis(2,3,4-trihydroxy-5-(N-4-butyric acid methyl sulfonamide)benzamide)(lyz003-194)
[0038]
[0039] The preparation method was as in Example 1, prepared from compound 2 (800mg, 2.57mmol), methyl 3-aminobutyrate hydrochloride (1.18g, 7.72mmol), p-phenylenediamine (48.7mg, 0.45mmol). Yellow-white solid with a yield of 67%.
[0040] 1 H NMR (400MHz, DMSO-d 6 )δ13.04(brs,1H),10.63(s,1H),8.05(s,1H),7.67(d,J=3.2,2H),3.93(s,1H),3.53(s,3H),2.83 –2.76(s,2H),2.36–2.26(m,2H),1.69–1.56(m,2H). 13 C NMR (100MHz, DMSO-d 6 )δ174.62,174.59,173.51,173.48,168.32,167.51,154.88,154.11,148.68,148.37,139.73,134.59,133.88,124.66,122.60,122.47,120.59,119.75,118.74,111.60,110.00,107.38,51.71,51.69,42.39 ,42.22,31.20,30.76,25.12,25.00.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com