Application of rifamycin-quinazine ketone coupling molecule
A technology of rifamycin and quinazinone, applied in the field of medicine, can solve problems such as side effects, low success rate, and reduced drug efficacy
Inactive Publication Date: 2018-07-24
TENNOR THERAPEUTICS (SUZHOU) LTD
View PDF3 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
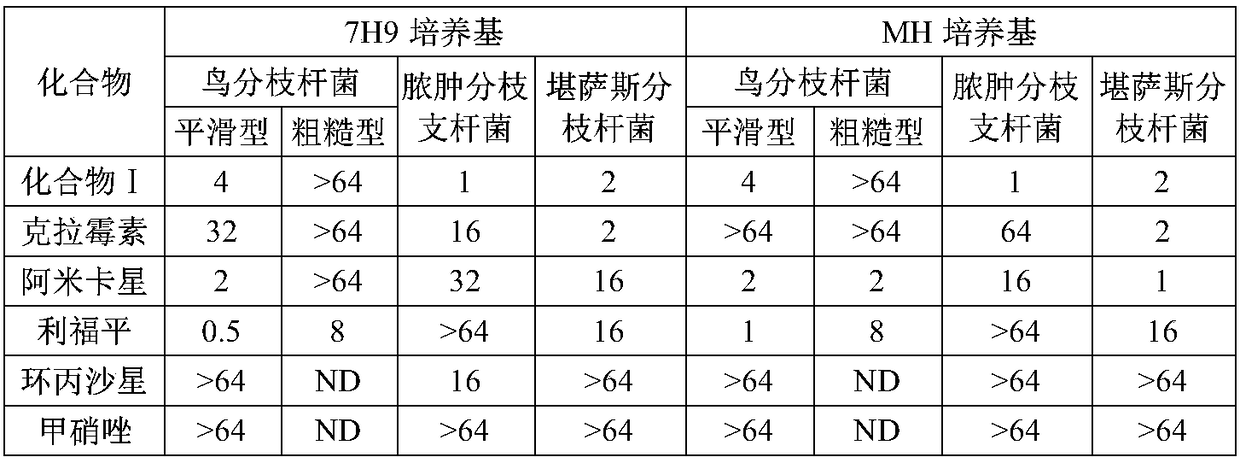
Due to the different species of mycobacteria that cause NTM infection, with different growth rates and characteristics, and different resistance to antimicrobial drugs, long-term antimicrobial treatment is often required, with low success rate and significant side effects
Especially in recent years, the resistance of NTM to existing drugs has been increasing, and the efficacy of existing drugs has been further reduced
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
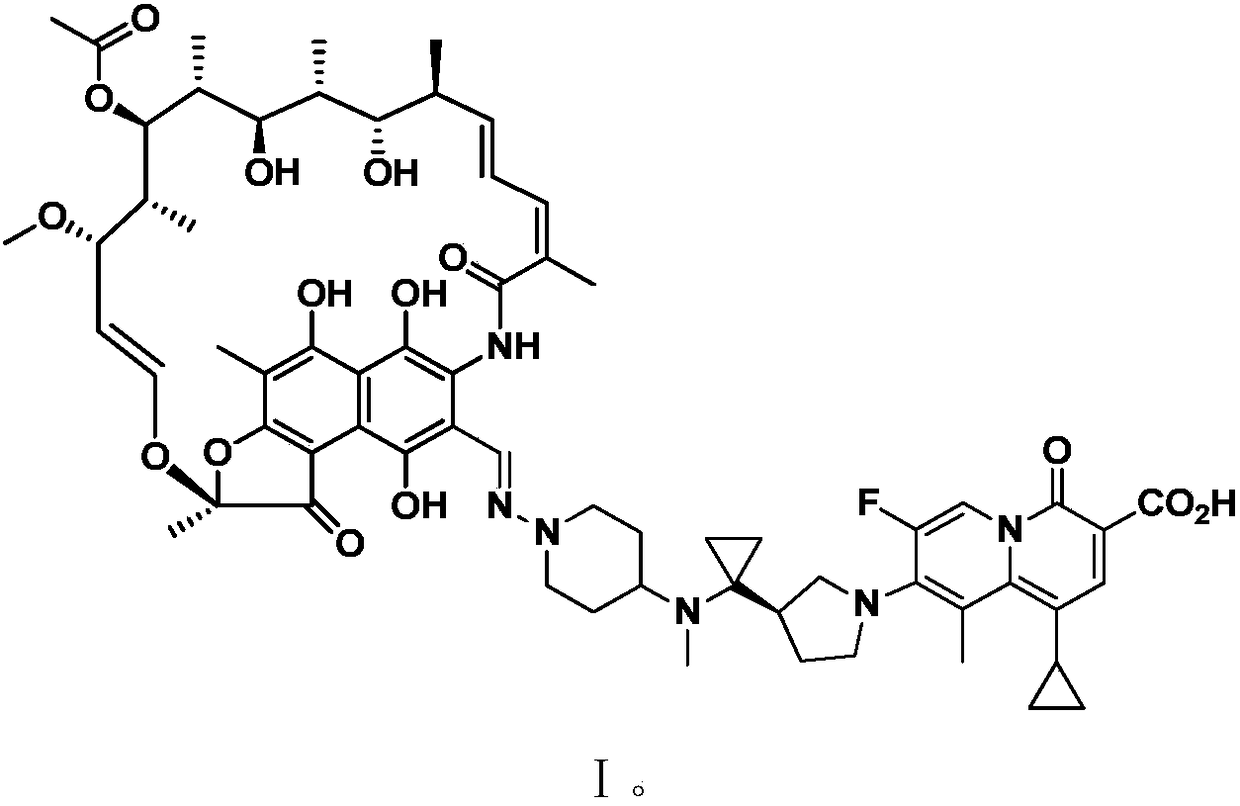
[0018] This example provides the application of a rifamycin-quinazinone conjugate molecule in anti-NTM. The rifamycin-quinazinone conjugate molecule has the structure shown in formula I:
[0019]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses application of a rifamycin-quinazine ketone coupling molecule or a three-dimensional isomer thereof or hydrate thereof or a deuterated material thereof, ester thereof, a solvent compound thereof, a crystal form thereof, a metabolic product thereof or pharmaceutically acceptable salt thereof or a pro-drug thereof in resisting nontuberculous mycobacteria. The rifamycin-quinazine ketone coupling molecule has a structure as shown in formula I. The rifamycin-quinazine ketone coupling molecule disclosed by the inention or the three-dimensional isomer thereof or hydrate thereof or the deuterated material thereof, ester thereof, the solvent compound thereof, the crystal form thereof, the metabolic product thereof or the pharmaceutically acceptable salt thereof or the pro-drug thereof can effectively resist nontuberculous mycobacteria, and can be used for treating infection caused by human nontuberculous mycobacteria.
Description
technical field [0001] The invention relates to the application of a rifamycin-quinazinone coupling molecule, which belongs to the technical field of medicine. Background technique [0002] Non-tuberculous mycobacteria (NTM), also known as atypical mycobacteria, refers to bacteria other than mycobacterium tuberculosis complex (MTC) and Mycobacterium leprae (Mycobacterium leprae). of mycobacteria. There are many ways to classify mycobacteria. From the perspective of clinical guidance, NTM can be simply divided into rapidly growing mycobacteria (RGM) and slowly growing mycobacteria (SGM). Medication choices provide useful information. Given the popularity of solid cultures, this sorting method can be implemented without special techniques and additional manipulations, making it practical. RGM can obtain macroscopic colonies within 3-7 days on solid medium, while SGM needs several weeks. The most common clinically valuable RGMs include Mycobacterium abscessus, Mycobacterium...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K47/55A61K31/395A61K31/4375A61P31/04
CPCA61K31/395A61K31/4375A61K2300/00
Inventor 马振坤袁鹰刘宇王晓梅
Owner TENNOR THERAPEUTICS (SUZHOU) LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com