Application of flavonoid GL-V9 in preparation of anti-leukemia drugs
A technology of flavonoids and GL-V9, applied in the field of application of flavonoids GL-V9 in the preparation of anti-leukemia drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
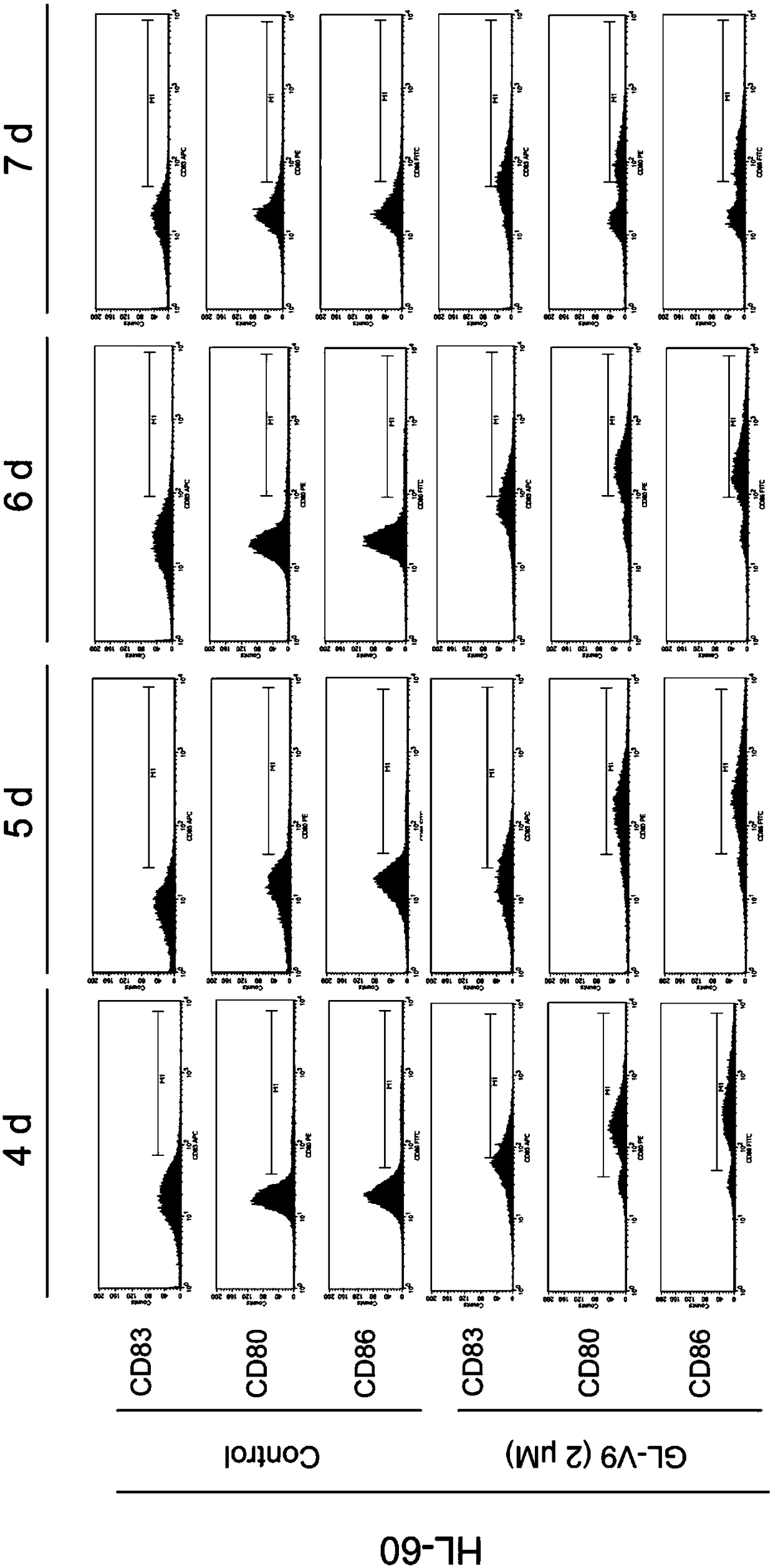
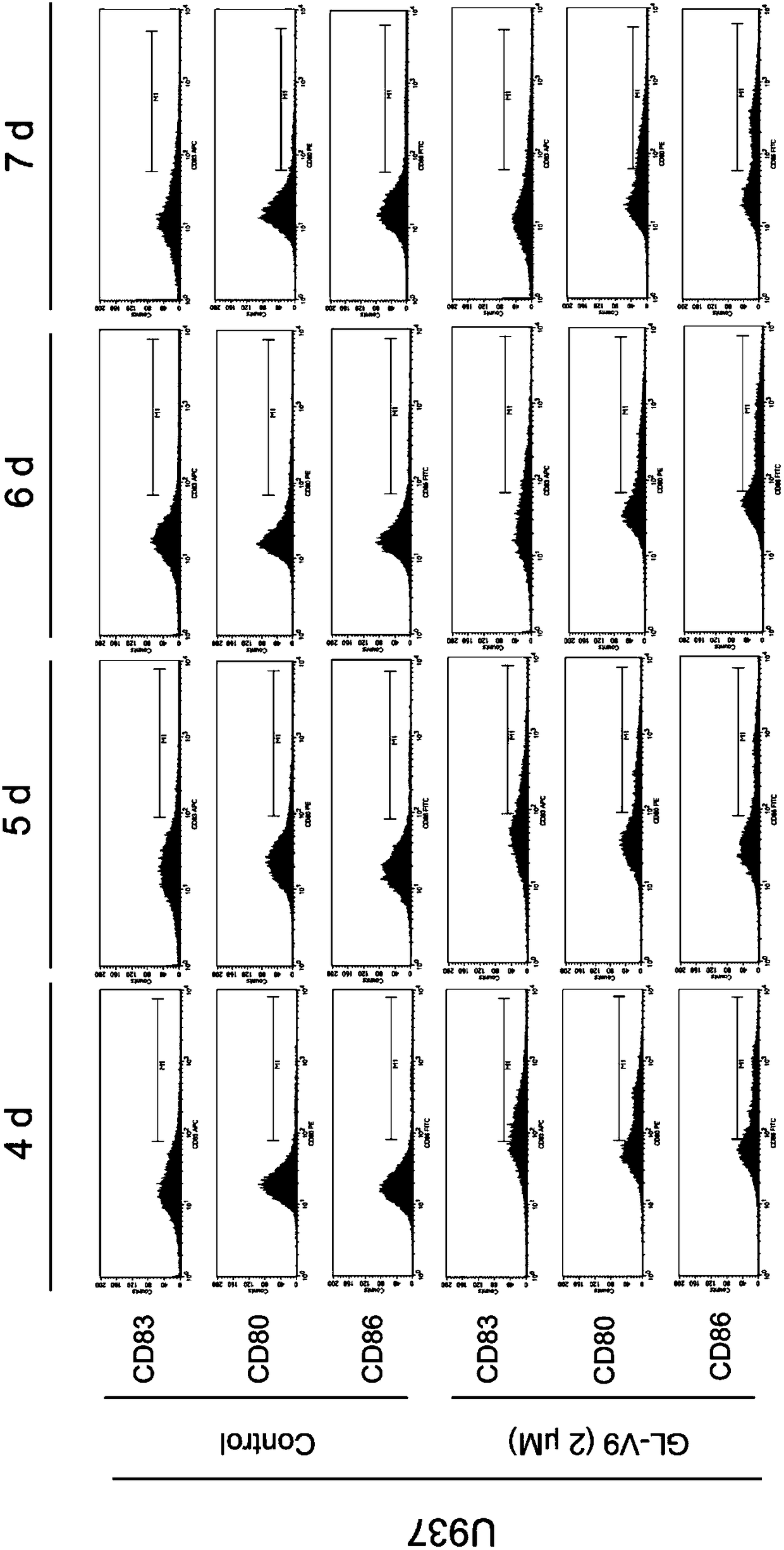
[0077] Example 3: Effect of GL-V9-induced DC-like cells produced by AML cells on secreted factors after co-culture with T cells.
[0078] 1. Experimental materials
[0079] 1.1 Reagents
[0080] (1) Drugs
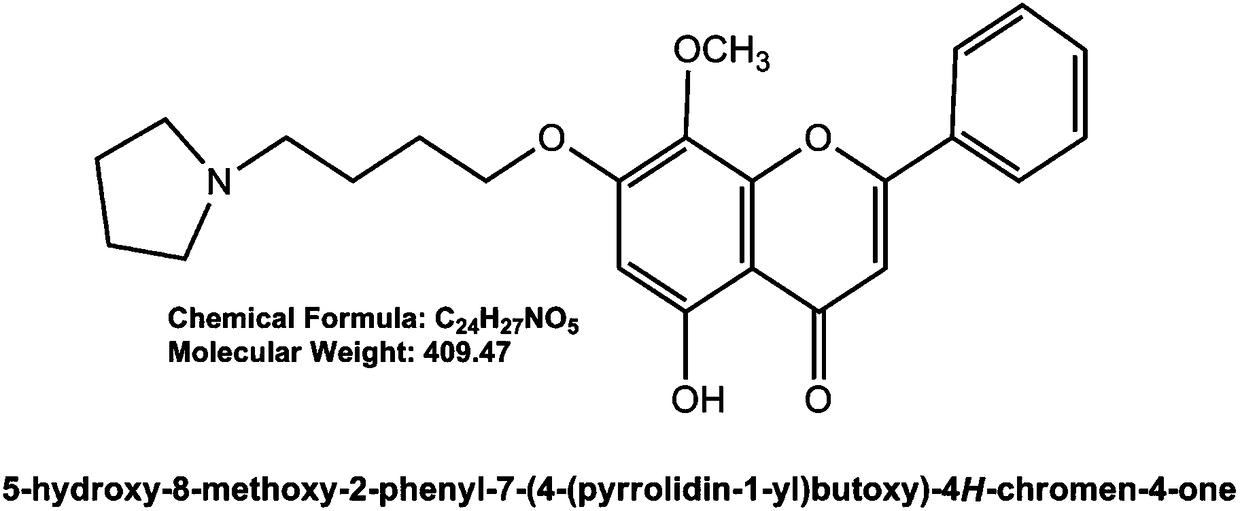
[0081] GL-V9(C 24 h 27 o 5 N, molecular weight: 409.47) was purchased from China Pharmaceutical University, a light yellow powder with a purity greater than 99%. Before use, the drug powder was prepared into a mother solution with a concentration of 0.02M with dimethyl sulfoxide (DMSO), and stored at -20°C. Prepare the required concentration with RPMI-1640 culture medium containing 10% fetal bovine serum before use
[0082] (2) Cell culture reagents
[0083] Culture medium: RPMI-1640 medium, purchased from GIBCO, USA. Take 10.39g of RPMI-1640 powder and dissolve it in 1000ml sterile three-distilled water, and add 2.0g NaHCO 3 , adjust the pH value to 7.0 with 1M hydrochloric acid, filter and sterilize with a cylindrical filter, pack in aliquots, and store in a refri...
Embodiment 1
[0110]Take 10g of GL-V9, add appropriate auxiliary materials for injection (including freeze-dried powder injection and sterile subpackaged dry powder injection), and prepare 1000ml of injection according to the injection (including freeze-dried powder injection and sterile subpackaged dry powder injection) process.
Embodiment 2
[0112] Take 10g of GL-V9, add appropriate excipients for tablets (including sustained and controlled release tablets, matrix tablets, coated tablets, dispersible tablets, etc.), press the tablet (including sustained and controlled release tablets, matrix tablets, coated tablets, dispersible tablets, etc.) ) process is prepared into 1000 tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com